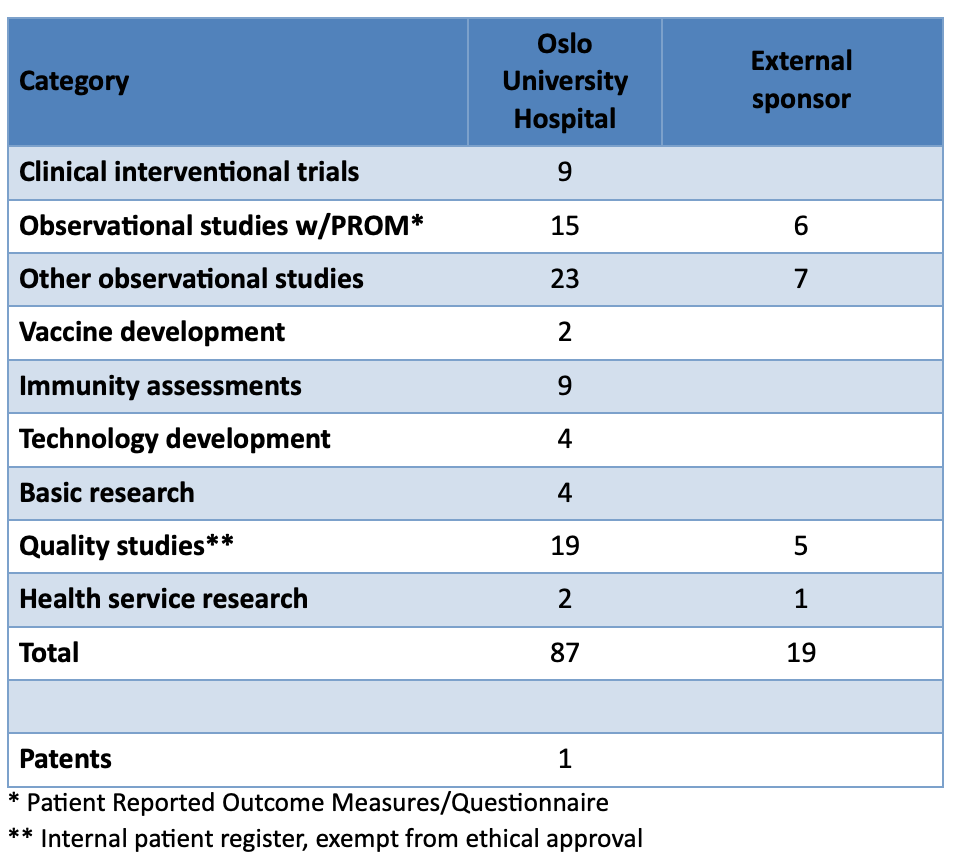
|
|
Project leader
|
Project name
|
Type of study
|
Ethics approval
|
Biobank
|
|
1
|
Anne Hege Aamodt
|
Division of Clinical Neuroscience
|
The Norwegian NeuroCOVID study
|
Multicenter prospective observational study
|
Conditional approval
|
Own collection
|
|
2
|
Anne Hege Aamodt
|
Division of Clinical Neuroscience
|
Compicated headache after COVID-19 vaccination
|
Multicenter observational study
|
Conditional approval
|
No
|
|
3
|
Hanne Birgit Alfheim
|
Division of Emergencies and Critical Care
|
Corona virus-related visitation restrictions enforced in ICUs in the Scandinavian countries – what can we learn to meet future challenges?
|
Survey
|
N/A
|
No
|
|
4
|
Erik Koldberg Amundsen
|
Division of Laboratory Medicine
|
Laboratory diagnostics for risk stratification in COVID-19
|
Observational study.
Laboratory based study
|
Approved
|
Own collection
|
|
5
|
Anders Åsberg
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
COVID-19 outcome in patients with chronic kidney disease
|
Epidemiological study, registry study, Diagnostic/Prognostic study
|
Approved
|
Other biobank
|
|
6
|
Anders Åsberg
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
Pharmacokinetic population analysis of antiviral drugs in the NOR SOLIDARITY study
|
Pharmacokinetic population modelling
|
Conditional approval
|
Own collection
|
|
7
|
Andreas Barratt-Due
Pål Aukrust
Anne Ma Dyrhol Riise
|
Division of Emergencies and Critical Care
Division of Surgery, Inflammatory Diseases and Transplantation
Division of Medicine
|
WHO: The NOR Solidarity multicenter trial on the efficacy of different anti-viral drugs in SARS CoV-2 infected patients
Clinical Trials.gov
|
Multicenter prospective clinical trial
|
Approved
|
Own collection
|
|
8
|
Andreas Barratt-Due
|
Division of Emergencies and Critical Care
|
Veno-venous ECMO-treatment of patients with COVID-ARDS
|
Quality study
|
N/A
|
No
|
|
9
|
Andreas Barratt-Due
|
Division of Emergencies and Critical Care
|
Handling and treatment of pregnant COVID-19 ICU patients
|
Quality study
|
N/A
|
No
|
|
10
|
Kristin Bjordal
Cecilie Delphin Amdal
|
Oslo Hospital Service
Division of Cancer Medicine
|
Development of an international questionnaire to assess patient-reported symptoms and concerns related to COVID-19 disease, the COVID-19 QLQ-##
|
Questionnaire development
|
Approved
|
No
|
|
11
|
Atle Bjørnerud
|
Division of Radiology and Nuclear Medicine
|
Artificial intelligence to quantify important segments from CT in COVID-19
|
Innovation
|
Under revision
|
No
|
|
12
|
Elisabeth Bø
|
Division of Medicine
|
Physiotherapy treatment of hospitalised patients with COVID-19 at Oslo University Hospital
|
Health service research
|
N/A
|
No
|
|
13
|
Marion Ingeborg Boldingh
|
Division of Clinical Neuroscience
|
Sars-CoV2 vaccine response in myasthenia patients
|
Observational study
|
Approved
|
COVID-19 general biobank
|
|
14
|
Arne Broch Brantsæter
|
Division of Medicine
|
Development of an antibody reference standards for COVID-19
|
Collection of plasma samples for vaccine development
|
Conditional approval
|
Own collection
|
|
15
|
Michael Bretthauer
Mette Kalager
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
Rapid-Cycle Re-Implementation of TRAining Facilities in Norway (TRAiN study)
|
Observational study
|
Approved
|
No
|
|
16
|
John Arne Dahl
|
Division of Laboratory Medicine
|
PREDCOV
|
Observational study
|
Approved
|
Yes
|
|
17
|
Veselka Petrova Dimova-Svetoslavova
|
Division of Laboratory Medicine
|
Establishment of Interferon-gamma-analysis for accurate detection of SARS-CoV-2 T-cell response
|
Basic research
|
N/A
|
No
|
|
18
|
Lars Eide
|
Division of Laboratory Medicine
|
COVID-19 general biobank
|
General research biobank
|
Approved
|
COVID-19 general biobank
|
|
19
|
Ane Gerda Z Eriksson
|
Division of Cancer Medicine
|
Influence of the COVID-19 pandemic on women with gynecological cancer
|
Quality study
|
N/A
|
No
|
|
20
|
Stig Evensen
|
Division of Mental Health and Addiction
|
COPSYC-19: Consequences for patients with psychosis from the SARS-CoV-2 shut-down
|
Observational study and patient survey
|
Approved
|
No
|
|
21
|
Ann Færden
|
Division of Mental Health and Addiction
|
The COVID-19 pandemic: Reactions of acutely ill psychiatric patients
|
Quality study
|
N/A
|
No
|
|
22
|
Ann Færden
|
Division of Mental Health and Addiction
|
The COVID-19 pandemic: reactions from mental health workers in the acute psychiatric department
|
Quality study
|
N/A
|
No
|
|
23
|
Yngvar Fløisand
Hanne Scholz
|
Division of Cancer Medicine
Division of Surgery, Inflammatory Diseases and Transplantation
|
Decidual stromal cells (DSC) in treatment of COVID-19 induced respiratory failure
|
Clinical trial
|
Conditional approval
|
Own collection
|
|
24
|
Tine Kristin Grimholt
|
Division of Medicine
|
COVID-19 and reactions in the Norwegian population
|
Survey
|
Approved
|
No
|
|
25
|
Tine Kristin Grimholt
|
Division of Medicine
|
Mental reactions to COVID-19 among adults with heart failure
|
Survey
|
Approved
|
No
|
|
26
|
Gunnveig Grødeland
|
Division of Laboratory Medicine
|
Novel vaccine development against COVID-19
|
Innovation project
|
N/A
|
No
|
|
27
|
Jostein Skjalg Hagemo
|
Divison of Prehospital Services
|
Prehospital treatment of "silent hypoxia" in young patients COVID-19
|
Case series
|
Conditional approval
|
No
|
|
28
|
Nina Eide Hasselberg
|
Division of Cardio-vascular and Pulmonary Diseases
|
Myocarditis and pericarditis after COVID-19 mRNA vaccination
|
Observational study
|
Conditional approval
|
No
|
|
29
|
Ansgar Heck
|
Division of Medicine
|
COVID-19 in patients with pituitary disease
|
Multicenter observational study
|
Conditional approval
|
Own collection
|
|
30
|
Åslaug Helland
|
Division of Cancer
|
SARS-CoV-2 and Cancer
|
Epidemiological observational study
|
Approved
|
No
|
|
31
|
Fridtjof Heyerdahl
|
Division of Prehospital Services
|
Inhaled NO as a bridge to rapid sequence intubation in COVID-19 related hypoxemia (INOCOV-19)
|
Randomized Controlled Trial
|
Conditional approval
|
No
|
|
32
|
Kristin Hofsø
|
Division of Emergencies and Critical Care
|
Survival rates and long-term outcomes for patients with COVID-19 admitted to Norwegian ICUs
|
Multicenter observational study
|
Approved
|
No
|
|
33
|
Aleksander Rygh Holten
|
Division of Medicine
|
VHP + 1836: EU-SolidAct - Baricitinib. A randomized, controlled multicenter study of hospitalized COVID-19 patients
|
Randomized Controlled Trial
|
Approved
|
No
|
|
34
|
Jan Cato Holter,
Susanne Dudman
Anne Ma Dyrhol-Riise
Aleksander Rygh Holten
|
Division of Laboratory Medicine
Division of Medicine
|
Norwegian SARS-CoV-2 study
Clinicaltrials.gov
|
Multicenter observational study
|
Approved
|
Own collection
|
|
35
|
Eivind Hovig
|
Division of Cancer Medicine
|
Production of "in house" versions of reverse transcriptase and Taq DNA polymerase for use in SARS-CoV-2 assays
|
Innovation project
|
N/A
|
No
|
|
36
|
Håvard Kalvøy
|
Oslo Hospital Service
|
Splitting one ventilator for multiple patients
|
Technical assessment
|
N/A
|
No
|
|
37
|
Tom Hemming Karlsen
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
Genetic risk for severe lung affection during COVID-19
|
Case-control association study
|
Conditional approval
|
COVID-19 general biobank
|
|
38
|
Per Kristian Knudsen
|
Division of Paediatric and Adolescent Medicine
|
Best treatment of multisystem inflammatory syndrome with COVID-19 (MIS-C)
|
Multicenter observational study
|
Conditional approval
|
No
|
|
39
|
Arne Kolstad
|
Division of Cancer Medicine
|
Monitoring of immunological response to COVID-19 vaccine in lymphoma/leukemia patients treated with anti-CD20 monoclonal antibodies and in patients who have completed allogenic stem cell transplantation
|
Observational study
|
Conditional approval
|
Own collection
|
|
40
|
Maria Stylianou Korsnes
|
Division of Mental Health and Addiction
|
COVID-19 impact on patient care
|
Quality study
|
N/A
|
No
|
|
41
|
Elfrida Hartveit Kvarstein
Geir Pedersen
|
Division of Mental Health and Addiction
|
Vulnerable patients with personality disorder - consequences of the corona crisis
|
Cross sectional, multi-center survey
|
Approved
|
No
|
|
42
|
Nina M. Kynø
|
Division of Paediatric and Adolescent Medicine
|
Pandemic 2020 - Parental debut in isolation
|
Survey
|
N/A
|
No
|
|
43
|
Jon Henrik Laake
|
Division of Emergencies and Critical Care
|
Handling oxygenation targets in adults with COVID-19 associated acute hypoxaemic respiratory failure in the ICU
|
Multicenter clinical trial
|
Approved
|
No
|
|
44
|
Irene Lie
|
Division of Cardiovascular and Pulmonary Diseases
|
A Norwegian study on views and experiences of health care professions working in intensive care units during the COVID-19 pandemic
Clinicaltrials.gov
|
Multicenter cohort and focus group interview study
|
Conditional approval
|
No
|
|
45
|
Tom Mala
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
Abdominal pain in COVID-19 patients
|
Case reports
|
Conditional approval
|
No
|
|
46
|
Karsten Midtvedt
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
COVID IgG KTx
|
Multicenter observational study
|
Conditional approval
|
Own collection
|
|
47
|
Karsten Midtvedt
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
SARS-CoV-2 Immune KTx-study
|
Multicenter observational study
|
Approved
|
Own collection
|
|
48
|
Peter Monrad-Hansen
|
Division of Emergencies and Critical Care
|
Acute appendicitis at Oslo University Hospital before and after Covid-19 restrictions: Business as usual or delay?
|
Quality study
|
N/A
|
No
|
|
49
|
Bjørn Moum
|
Division of Medicine
|
Secure IBD registry. Surveillance epidemiology of Coronavirus (COVID-19) under research exclusion.
|
Quality study
|
N/A
|
No
|
|
50
|
Ludvig Munthe
|
Division of Laboratory Medicine
|
Defining the immune cells that correlate with fatal acute lung injury in COVID-19
|
Epidemiological pathogenetic study
|
|
COVID-19 general biobank
|
|
51
|
Ludvig Munthe
|
Division of Laboratory Medicine
|
Establishing in tube-diagnostic tests for immunity or susceptibility for SARS-CoV-2 in elderly cancer patients and in high-risk children
|
Innovation project
|
N/A
|
No
|
|
52
|
Lise Sofie Nissen-Meyer
|
Division of Laboratory Medicine
|
Convalescent plasma therapy for patients with severe COVID-19
|
Clinical trial
|
Conditional approval
|
COVID-19 general biobank
|
|
53
|
Lise Sofie Nissen-Meyer
|
Division of Laboratory Medicine
|
NORPLASMA monitor
|
Observational study
|
Conditional approval
|
No
|
|
54
|
Lise Sofie Nissen-Meyer
|
Division of Laboratory Medicine
|
Antibody screening in blood donors
|
Observational study
|
Approved
|
COVID-19 general biobank
|
|
55
|
Lise Sofie Nissen-Meyer
|
Division of Laboratory Medicine
|
Novel antibodies against HLA1
|
Observational study
|
N/A
|
COVID-19 general biobank
|
|
56
|
Ingvild Nordøy
|
Division of Surgery, Inflammatory Diseases and Transplantation
|
Superinfections in Covid-19 patients in the ICU - before and after dexamethasone
|
National multicenter retrospective observational study
|
Approved
|
No
|
|
57
|
Gro Owren Nygaard
|
Division of Clinical Neuroscience
|
NevroVAX
|
Quality study
|
N/A
|
No
|
|
58
|
Randi Opheim
|
Division of Medicine
|
Views of patients with inflammatory bowel disease on the COVID-19 pandemic and the health care service
|
Quality study
|
N/A
|
No
|
|
59
|
Karl Øyri
|
Division of Emergencies and Critical Care
|
Research and Development for a low-cost, quickly produced mechanical ventilator capacity scaling system for COVID-19 affected communities
|
Technical assessment
|
N/A
|
No
|
|
60
|
Frank Olav Pettersen
|
Division of Medicine
|
SARS-CoV-2 antibody screening in health care professionals at OUS
|
Quality study
|
N/A
|
No
|
|
61
|
Frank Olav Pettersen
|
Division of Medicine
|
SARS-CoV-2 among hospital staff at Oslo University Hospital
|
Quality study
|
N/A
|
No
|
|
62
|
Frank Olav Pettersen
|
Division of Medicine
|
Incidence and characteristics of SARS-CoV-2 immune response among first line COVID-19 hospital staff
|
Observational study
|
Approved
|
COVID-19 general biobank
|
|
63
|
Søren Pischke
|
Division of Emergencies and Critical Care
Division of Laboratory Medicine
|
Prevalence of complement activation in COVID-19 patients admitted to OUS
|
Quality study
|
N/A
|
No
|
|
64
|
Marius Rehn
|
Division of Prehospital Services
|
Pre-hospital management of COVID-19
|
Quality study
|
N/A
|
No
|
|
65
|
Anne Ma Dyrhol-Riise
|
Division of Medicine
|
A Phase 1/2 Study to Determine Safety and Immunogenicity of Two COVID 19 Vaccines VB10.2129 (RBD Candidate) and VB10.2210 (T Cell Candidate) Previously Vaccinated in Healthy Adult Volunteers
Clinicaltrials.gov
|
Clinical trial
|
Approved
|
No
|
|
66
|
Kristin Lie Romm
|
Division of Mental Health and Addiction
|
REACT-NOR - react now!
Remote follow-up of relatives of patients with psychosis during the COVID-19 crisis
|
Health service research
|
N/A
|
No
|
|
67
|
Kristin Lie Romm
|
Division of Mental Health and Addiction
|
COPE - Corona pandemic experiences by patients with psychotic - and bipolar disorders
|
Survey
|
Approved
|
No
|
|
68
|
Ellen Ruud
|
Division of Paediatric and Adolescent Medicine
|
Effect of the COVID19 pandemic on pediatric cancer incidence at Oslo University Hospital
|
Quality study
|
N/A
|
No
|
|
69
|
Nina Haagenrud Schultz
|
Division of Cancer Medicine
|
Venous thrombosis after COVID-19 vaccines
|
Observational study
|
Conditional approval
|
Own collection
|
|
70
|
Nina Haagenrud Schultz
|
Division of Cancer Medicine
|
Do COVID-19 vaccines lead to increased coagulation ability?
|
Observational study
|
Conditional approval
|
Own collection
|
|
71
|
Andreas Seierstad
|
Division of Mental Health and Addiction
|
COVID-19 and labor market participation for young adults with psychosis
|
Observational study
|
N/A
|
No
|
|
72
|
Vasilis Sitras
|
Division of Paediatric and Adolescent Medicine
|
COVID-19 in Pregnancy, Childbirth and Puerperium (COVID-Preg-ChiP Study)
|
Observational study with survey
|
Conditional approval
|
Own collection
|
|
73
|
Anne Lee Solevåg
|
Division of Paediatric and Adolescent Medicine
|
Visitor bans in hospitals: How are newborn infants affected during the COVID-19 pandemic?
|
Observational study with survey
|
Conditional approval
|
Own collection
|
|
74
|
Arne Vasli Søraas
|
Division of Laboratory Medicine
|
Risk factors for community- and workplace transmission of COVID-19
Clinical Trials.gov
|
Epidemiological observational study
|
Approved
|
No
|
|
75
|
Arne Vasli Søraas
|
Division of Laboratory Medicine
|
Cod Liver Oil for COVID-19 Prevention Study
|
Non-clinical interventional study
|
Approved
|
Other biobank
|
|
76
|
Ketil Størdal
|
Division of Paediatric and Adolescent Medicine
|
COVID-19 and children in BEREDT-C19
|
Epidemiological study
|
N/A
|
No
|
|
77
|
Eva Stylianou
|
Division of Medicine
|
Allergological evaluations of corona vaccination
|
Quality study
|
N/A
|
No
|
|
78
|
Kjetil Sunde
|
Division of Emergencies and Critical Care
|
Intensive medicine priority decisions during the COVID-19 pandemic (INTENSIVE TRIAGE)
|
Prospective multicentre observational study
|
Approved
|
No
|
|
79
|
Kjetil Taskén
|
Division of Cancer Medicine
|
Immunoregulatory and antiviral properties of compounds derived from anti-malaria drugs as hydroxychloroquine
|
Explorative preclinical drug development
|
N/A
|
No
|
|
80
|
Kjetil Taskén
|
Division of Cancer Medicine
|
Aminoacridines with dual SARS-CoV2 antiviral and immunostimulating activity
|
Explorative preclinical drug development
|
N/A
|
No
|
|
81
|
Suraj Bahadur Thapa
|
Division of Mental Health and Addiction
|
Psychological distress and Coping in Psychiatric Patients during COVID-19 pandemic (PsyCo-COVID-19): A cross-sectional study at Oslo University Hospital
|
Survey
|
Conditional approval
|
No
|
|
82
|
Kirsti Tøien
|
Division of Emergencies and Critical Care
|
Visiting restrain to hospitals; family caregivers of COVID-19 ICU-patients experiences. An exploratory study
|
Survey
|
N/A
|
No
|
|
83
|
Kristian Tonby
Theresa Olasveengen
Aleksander Rygh Holten
|
Division of Medicine
Division of Emergencies and Critical Care
Division of Medicine
|
COVID-19 OUS. Kvalitetsregister for alle pasienter innlagt ved Oslo Universitetssykehus med COVID-19 sykdom.
|
Quality study
|
N/A
|
No
|
|
84
|
Kristian Tonby
|
Division of Medicine
|
Korona-screen
|
Quality study
|
N/A
|
No
|
|
85
|
Kristian Tonby
|
Division of Medicine
|
Treatment of hospitalized COVID-19 – A randomized multicenter study (Discovery)
|
Clinical trial
|
Approved
|
No
|
|
86
|
Margrethe von Tangen
|
Division of Emergencies and Critical Care
|
Retrospektiv vurdering av triagering av Covid-19 pasienter ved bruk av nytt Kap 41: Mistanke om Corona-infeksjon
|
Quality study
|
N/A
|
No
|
|
87
|
Lars Wik
|
Division of Prehospital Services
|
Sensor based vital signs monitoring of patients with clinical manifestation of COVID-19 disease during home isolation
|
Randomized feasibility study
|
Conditional approval
|
No
|