How B cell receptor ligation and T cell help drives chronic pathological B cell expansion

Scientists at the University of Oslo and Oslo University Hospital have recently published a paper in Proceedings of the National Academy of Sciences of the United States of America (PNAS) that might explain why a particular type of immune response can cause development of autoimmunity and even cancer:
B cell receptor ligation induces display of V-region peptides on MHC class II molecules to T cells
Peter Csaba Huszthy, Ramakrishna Prabhu Gopalakrishnan, Johanne Tracey Jacobsen, Ole Audun Werner Haabeth, Geir Åge Løset, Ranveig Braathen, Karl Schenck, Anders Aune Tveita, Ludvig Andre Munthe and Bjarne Bogen
Background and resume of the paper, in brief:
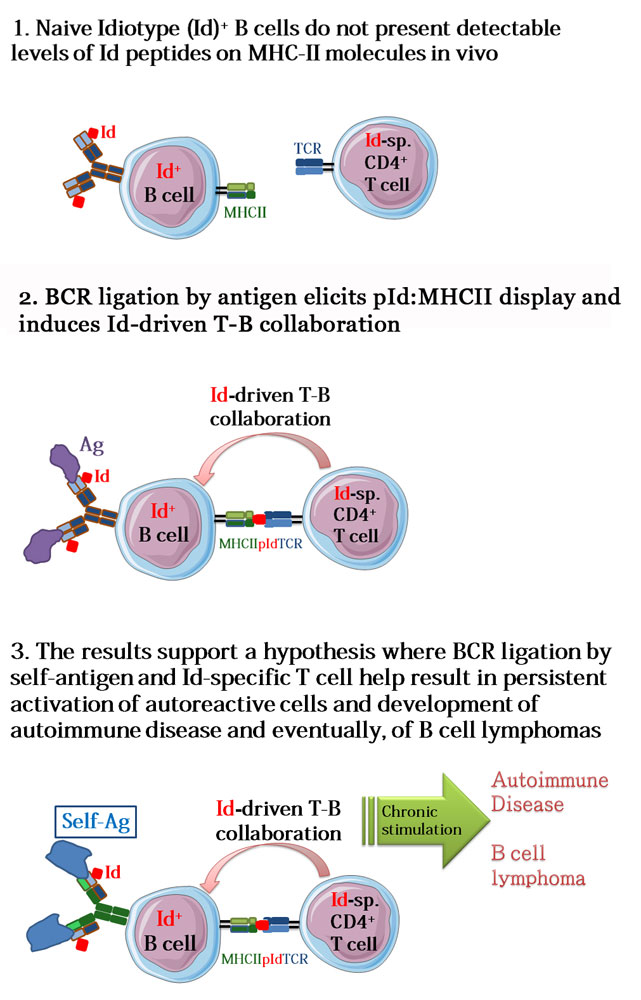
Activation of a particular class of white blood cells, B cells, by a foreign substance, e.g. a virus, results in the production of protective antibodies. The activation of the given B cell requires that it receives help from another type of white blood cells, T cells, which recognize parts of the virus when presented by the B cells. This happens in the following way: The B cell expresses a B cell receptor (BCR) that recognizes a viral antigen (most often, a protein). BCR-antigen complexes are taken up into the B cell, followed by a partial breakdown of the viral antigen. Next, short fragments of the viral antigen are bound to Major Histocompatibility Complex (MHC) class II molecules followed by export to the B cell’s surface where the Ag:MHCII complexes are recognized by antigen-specific T helper cells of the CD4 type. This mechanism is called conventional T-B collaboration and is well known to be involved in most antibody responses to foreign antigens. In the new work of Peter Huszthy et al., the authors demonstrate that when the BCR of a B cell binds antigen, the highly variable regions of the BCR are also degraded to short fragments that are presented on MHC class II molecules to T helper cells, which then help the B cells. The short fragments derived from the BCR variable regions are called idiotopes (Id), hence, the novel type of collaboration between B and T cells is called Id-driven T-B collaboration. The results contribute to our understanding of how Id-driven T-B collaboration can induce autoimmune diseases and even B cell cancers such as B cell lymphomas.
In depth description of the paper and its significance:
The present study significantly extends previous findings from immunologists at the University of Oslo and Oslo University Hospital. Starting from the second half of the 1980’s, investigators in the Bogen lab at the Dept. of Immunology have performed pioneering work to establish the concept of Id-driven T-B collaboration. Further, they showed that when established in mice, Id-driven T-B collaboration induces chronic expansion of Id+ B cells, resulting in the development of severe systemic autoimmune disease and even B cell lymphomas. Indications to that this process contributes to human pathology has been found in patients with multiple sclerosis (Høglund et al., Front Immunol., 2017) and in chronic lymphatic leukemia (Os et al., Cell Rep., 2013), both working at UiO/OUH. Recent work by Khodadoust et al. (Nature, 2017) at Stanford University demonstrates that MHCII presentation of Id-peptides is involved in the development of B cell cancers. However, the importance of binding of antigen to the BCR for initiation of Id-driven T-B collaboration has been unknown. An importance might be suspected because previous findings by others, both in mice and humans, have demonstrated that the BCRs of B cells involved in development of autoimmunity and B lymphoma bind self-antigens. This suggested to the authors of the present paper that binding of a B cell’s BCR to self antigen could initiate disease-causing Id-driven T-B collaboration. To demonstrate this, first author Peter Huszthy and colleagues in the Bogen lab established a first of its kind mouse model, where a native BCR light chain gene was replaced by its mutated counterpart that contained an idiotope. The mouse was made physiological by a targeted replacement of the light chain gene, whereby the mutated gene was allowed to undergo physiological BCR light chain recombination. After crossing these mice with another strain of mice that expressed the complementary BCR heavy chain, expression of the complete Id+ BCR was expressed on naïve B cells. Taking advantage of specific BCR ligands that are able to crosslink the Id+ BCR and a detection reagent that recognizes complexes of BCR-Id fragments presented on MHCII molecules, the authors showed that BCR ligation induces the expression of BCR-Id fragments on MHCII molecules, thereby enabling co-stimulatory interactions with Id-specific T helper cells (Id-driven T-B collaboration, see figure to the right). Id-driven T-B collaboration initiated by BCR ligation elicited germinal center formation, antibody production and memory B cell differentiation. The findings demonstrate the importance of BCR ligation in the process of Id-driven T-B collaboration, and provide an explanation for the self-reactivity of pathological, chronically expanding B cell populations in autoimmune disease and B cell lymphomas.
Links:
B cell receptor ligation induces display of V-region peptides on MHC class II molecules to T cells
Peter Csaba Huszthy, Ramakrishna Prabhu Gopalakrishnan, Johanne Tracey Jacobsen, Ole Audun Werner Haabeth, Geir Åge Løset, Ranveig Braathen, Karl Schenck, Anders Aune Tveita, Ludvig Andre Munthe, and Bjarne Bogen
PNAS first published December 3, 2019. https://doi.org/10.1073/pnas.1902836116
In Silico Prediction Analysis of Idiotope-Driven T-B Cell Collaboration in Multiple Sclerosis.
Høglund RA, Lossius A, Johansen JN, Homan J, Benth JŠ, Robins H, Bogen B, Bremel RD, Holmøy T.
Front Immunol. 2017 Oct 2;8:1255. doi: 10.3389/fimmu.2017.01255. eCollection 2017.
PMID: 29038659
Chronic lymphocytic leukemia cells are activated and proliferate in response to specific T helper cells.
Os A, Bürgler S, Ribes AP, Funderud A, Wang D, Thompson KM, Tjønnfjord GE, Bogen B, Munthe LA.
Cell Rep. 2013 Aug 15;4(3):566-77. doi: 10.1016/j.celrep.2013.07.011. Epub 2013 Aug 8.
PMID: 23933259
Antigen presentation profiling reveals recognition of lymphoma immunoglobulin neoantigens.
Khodadoust MS, Olsson N, Wagar LE, Haabeth OA, Chen B, Swaminathan K, Rawson K, Liu CL, Steiner D, Lund P, Rao S, Zhang L, Marceau C, Stehr H, Newman AM, Czerwinski DK, Carlton VE, Moorhead M, Faham M, Kohrt HE, Carette J, Green MR, Davis MM, Levy R, Elias JE, Alizadeh AA.
Nature. 2017 Mar 30;543(7647):723-727. doi: 10.1038/nature21433. Epub 2017 Mar 22.
PMID: 28329770