Stem cells and brain cancer
Background
Brain cancers in principle always recur despite apparent complete removal under the operating microscopie and subsequent adjuvant therapy. This is particularly true for the most common intracranial tumor type, the glioblastoma,where 50 percent of treated patients die within one year from diagnosis.
In parallel with results emerging from other research institutions, our group has shown that only a subpopulation of cells in brain cancers have the ability to proliferate and initiate new tumors following transplantation to immunodeficient mice. This cell population infiltrates surrounding brain tissue, appears resistant to both irradiation and chemotherapy, and is the likely explanation for recurrence.
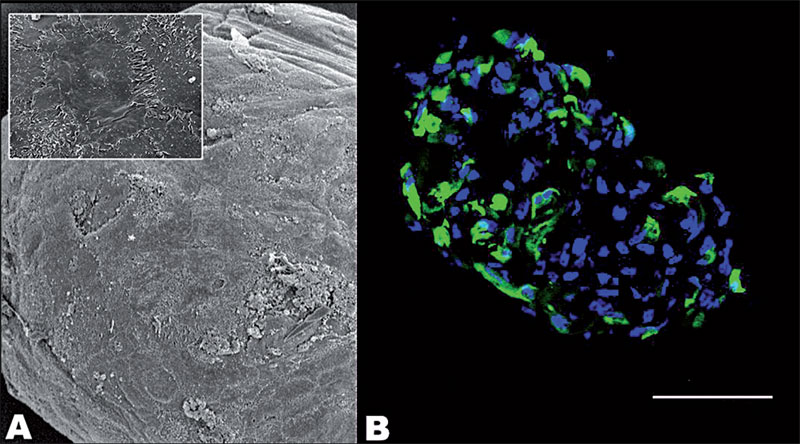 |
| Figure 3: Scanning electron micrographs of human ciliary body (CB) sphere (A). Immunostaining of human CB sphere with the neural stem cell marker nestin (green) (B). Magnification: A, 248X, inset 1811X; scalebar B, 40 um. |
Results
In a premier study, Mercy Varghese and Morten Moe showed that these cells share a number of the properties of normal neural stem cells of the adult human brain. Håvard Ølstørn demonstrated that stem cells isolated from tumors could reproduce the tumor in immunodeficient mice, whereas stem cells from normal human brain did not result in tumors (Fig. 2).
Both normal and tumor stem cells showed a high proliferative index when cultivated as such. Interestingly, the proliferative index fell dramatically also in tumor stem cells when they were induced to differentiate. Normal and tumor stem cells showed a similar pattern of differentiation, i.e. in neuronal and glial directions, although differentiated cells from the tumor were clearly abnormal morphologically and differentiation in itself progressed much faster. These results have been published in Neurosurgery, 2008 and have been presented in international conferences.
An important implication of the tumor stem cell concept is that one may direct treatment directly against the progenitor population (the proliferating subgroup of cells that cause recurrence). Einar Vik-Mo has performed a number of experiments studying the effect that in vitro culture of tumor stem cells has on the cells’ ability to form tumors, to
differentiate and to undergo genotypic and expressional changes. He is also exploring the cellular organization of neuro- and tumorspheres, looking at the cellular heterogeneity of such spheres. By sorting tumor cells based on surface antigens, we hope to establish methods for better identification of the progenitor population. In collaboration with Charles Liu and David Tirrell at USC/ Caltech we are exploring the effects of artificial extracellular matrix on the differentiation and proliferation of normal and tumor stem cells. Data from this work was presented at the International Society for Stem Cell Research 2008 and the Congress of Neurological Surgeons 2008.
We have also used this technology and experiences to establish a clinical protocol which was formally accepted by the necessary authorities during 2008. This protocol is designed to harness the patients’ own immunity. Patients will be recruited from January 2009. This protocol is backed up from the collaboration through the Cancer Stem Cell Innovation Center (SFI CAST) and is a collaboration with the Neurosurgical departments at Ullevål universitetssykehus and Rikshospitalet, Avd. for klinisk kreftforskning ,Avd. for celleterapi, and Avd. for immunologi, Institutt for kreftforskning, Radiumhospitalet and the oncological departments at both Radiumhospitalet and Ullevål universitetssykehus.
Cecilie Sandberg has compared the global gene expression in normal stem cells and tumor stem cells, in order to identify possible targets for treatment and to better understand the biology of the cell population that escapes current treatment and causes recurrences. The results of this comparison study show a significant upregulation in tumour stem cells of genes connected to regulation of focal adhesion,actin cytoskeleton, axon guidance as well as the Wnt pathway.Currently, the possible target genes are investigated at the protein level using immunohistochemistry and Western blot. The role of the possible targets in the Wnt pathway are investigated by Kirsten Strømme using siRNA-knockdown based technology. Cecilie Sandberg is also, in collaboration with Morten Moe, exploring the transcriptome of single cells in neuro- and tumorspheres, looking at the cellular hetereogenity of such spheres.
Aquaporins or water-channels are targets indicated by Cecilie’s work. Guri Fossdal has investigated the expression of these in tumor stem cells and their differentiated counterparts. This work is ongoing. Mrinal Joel is studying transplantation of GFP-transduced brain tumour stem cells to an embryonic environment using the chick embryo model. She investigates the behaviour and differentiation potential of tumour cells when placed in this environment. So far, interestingly, tumor cells display a more restricted activity in an embryonic environment compared to adult. Further studies will be based on the analyses of proliferation, cell death and differentiation ability of these cells into other cell types. Co-culture studies of the tumour cells with the cells from the central nervous system (CNS) of chick embryo are also under investigation to examine these effects.
Sandrine Pacchini is comparing normal human brain tissue-derived stem cells and different human brain tumor (glioblastoma and ependymoma) derived stem cells. This study investigates mismatches in gene expression between normal and tumor derived stem cells, differences in cell surface receptor expression, and survival and proliferation rates. Knowledge gained through these studies could contribute to more efficient therapeutic techniques, such as vaccines targeting cells within the tumors.
Post-Doc John Bianco is performing cell biology studies on tumor (glioblastoma) and normal adult neural stem cells, both brain-derived and from the adult olfactory mucosa, a clinically relevant, readily accessible source of adult neural stem cells. Initial studies are aimed at developing a unified culture method for the rapid isolation, maintenance and proliferation of undifferentiated adult stem cells. Many protocols have been developed, using various techniques, which try to answer some of the questions that have arisen so far regarding the biology and manipulation of stem cells in vitro, with the hope of expanding the knowledge gained in vivo in the form of cell based therapies for a wide range of pathologies. However, at present, a robust technique for the culture of these cells is still in development. Once this has been established, studies will be performed to assess the fundamental processes determining the fate of stem cells and their differentiation potential. In particular, differentiation into the neural lineages - namely oligodendrocytes, astrocytes, and neurons - will be analyzed. Directed differentiation of these cells towards a specific fate such as down the dopaminergic pathway to obtain dopaminergic neurons will also be examined. With this progression in stem cell research and some understanding of how to manipulate them in vitro, a new era of cell therapy of great potential is possible.
Proteomic studies to find surface markers in adult neural and cancer stem cells are being carried out by Post-Doc Linda Paulson. Currently, cancer is viewed by some as an aberrant organ with a self-renewing stem cell population. These cells are suggested to resemble stem cells from the normal human brain. Intrinsic brain tumours are usually malignant and resistant to treatment. The most common form, glioblastoma (astrocytoma grade IV), has a median survival of 12 months. Traditionally these tumours have been thought to be derived from the transformation and dedifferentiation of glial cells residing within the brain parenchyma. Recent evidence, however, indicates that these tumours consist of 1) a small population of cells that is able to divide, give rise to new cells and drive tumour growth (i.e. cancer stem cell), and 2) differentiated cells with either no or a diminished ability for cell division. This latter cell type represents the bulk of the tumour. These tumours are usually completely resected during surgery. Despite this they always recur. The reason for this is that the putative glioma stem cells have a strong capability to migrate into the normal brain and form satellites. At the time of diagnosis such cells have usually reached the opposite hemisphere. A cure for these patients will therefore depend on our ability to directly target the glioma stem cells. In the present study, we will compare protein surface markers from neural stem cells from the adult human brain (ANSCs) with glioma stem cells (GSCs) from astrocytma grade II. Despite similarities between GSCs and ANSCs, differences will be characterized that may be important when developing therapy specifically targeting the cancer stem cell. Linda is also studying the difference in protein degradation in brain tumors in snap frozen samples compared with samples that are heated to 95° C in vacuum.
Proteins start to degrade after only two minutes after tissue is removed and it is crucial to find ways to prevent this. When this study is completed and methods are optimized we are going to study all brain tumors removed during one year in order to classify the tumors according to their proteomic expression. This would help greatly in future treatment targets. Results are going to be presented at the HUPO 8th Annual World Congress, Toronto 2009
