Projects
|
EMIT: Establishment of Molecular profiling for Individual Treatment decisions in Early Breast Cancer |
||
|
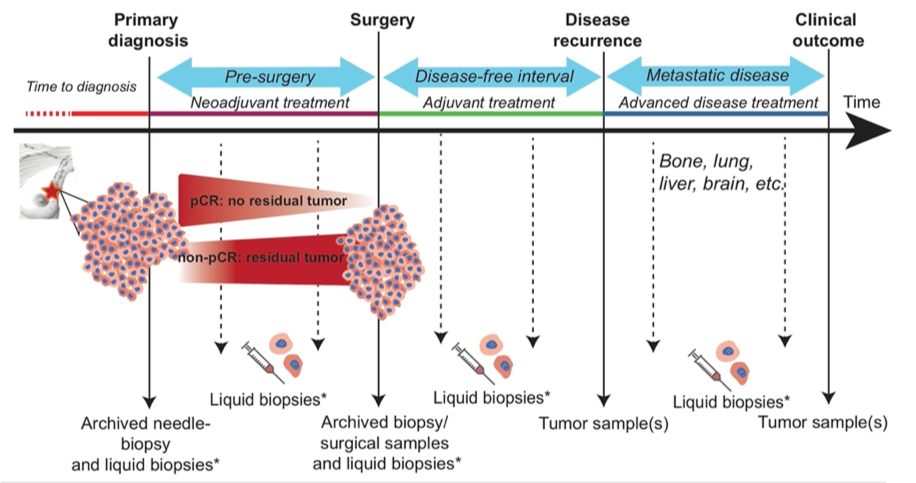
The project aims to optimize the use of adjuvant chemotherapy in early stage, hormone receptor positive breast cancer patients, by implementing a multi-parameter test (PAM50) for risk- and subtype-classification. |
 |
|
|
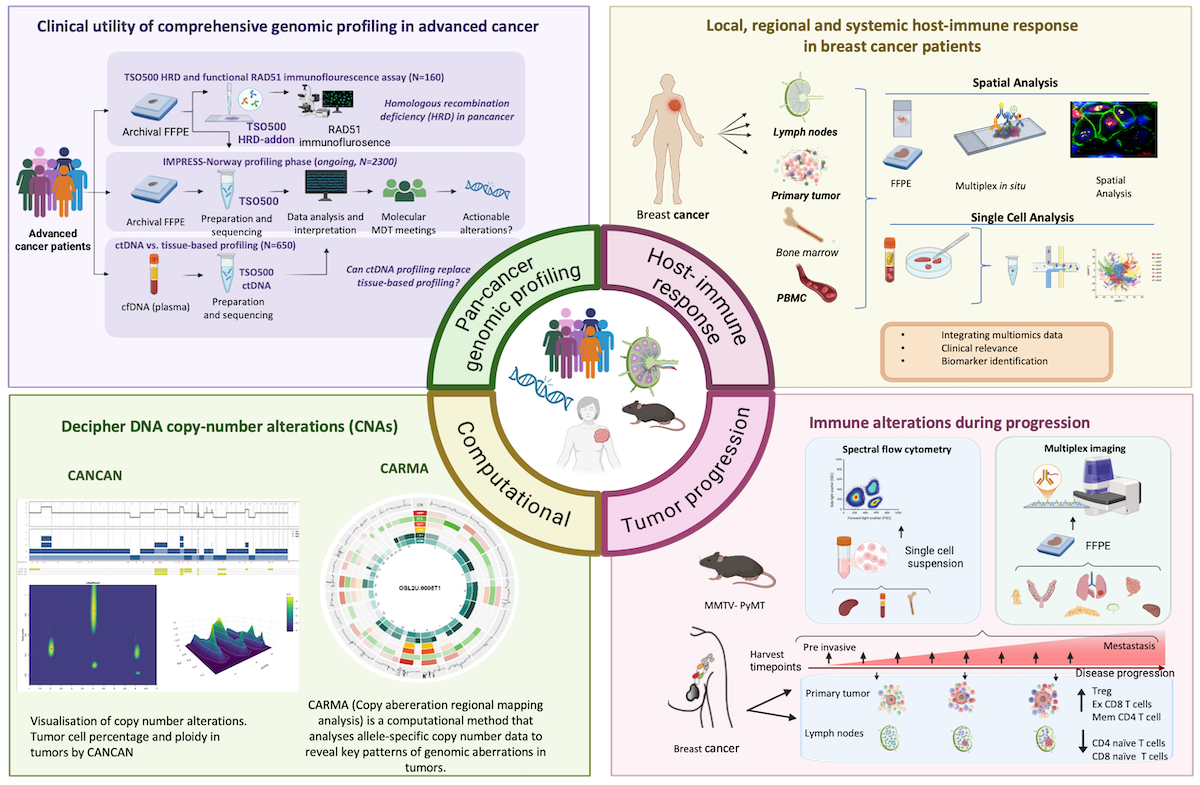
Circulating tumor DNA (ctDNA) as a liquid biopsy for advanced cancer patients |
||
|
IMPRESS-Norway is a national precision medicine clinical trial evaluating efficacy of targeted anti-cancer drugs for advanced cancer patients based on potentially actionable biomarkers revealed by molecular profiling. For patients included in IMPRESS-Norway, we will in this subproject perform comprehensive genomic profiling (CGP) using circulating tumor DNA (ctDNA) in plasma from peripheral blood to identify actionable biomarkers for treatment recommendation. The project aims to identify patient groups where CGP of ctDNA is beneficial compared to tissue biopsies, and evaluate if liquid biopsy analyses based on ctDNA can be implemented into the public health care system.
|
 |
|