The research program is a multidisciplinary effort within Oslo University Hospital, and includes partners from the Institute for Cancer Research, Department of Molecular Oncology and Department of Immunology (Kjetil Taskén lab), Department of Gynaecologic Oncology (Anne Dørum and Katharina Bischoff), and Department of Pathology (Ben Davidson). The subprojects of the Department of Molecular Oncology is run jointly by the Skotheim lab and Lothe lab with the following scientists involved: Bjarne Johannessen, Anita Sveen, Mie Jareid and Solveig Klokkerud.
Ovarian cancer (OC) is a global health challenge and is renowned as the most lethal among gynaecological cancers. Two-thirds of the OC patients have metastases already at diagnosis. Patients with localized disease have a five-year survival rate of 80-90%, but drastically lower at approximately 30% and 15% for those diagnosed with stage III and IV OC, respectively. Standard primary treatment of advanced OC has until recently been radical surgery with adjuvant or neoadjuvant platinum and paclitaxel chemotherapy. However, chemotherapy resistance and toxicity, often after repeated cycles with chemotherapy, is the main reason for treatment failure. Targeted therapy with patient benefit is first-line poly (ADP-ribose) polymerase (PARP) inhibitors. Aditionally, maintenance treatment with bevacizumab following a relapse of platinum-sensitive cancer prolongs the average progression-free survival. Epithelial OC is categorized into type I (~25%) and II (~75%). High grade serous OC is the most aggressive among type II cancers and almost all have mutations in the TP53 gene and about one in five in BRCA1/2.
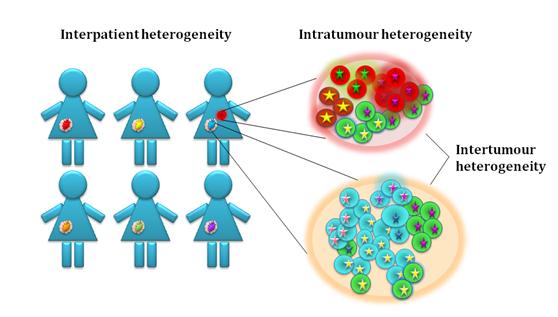
Knowledge of the molecular biology of OC is important to improve treatment and prolong survival. The aim of our research is to understand the clonal evolution, including the molecular heterogeneity of disease progression and chemoresistance. We compare inter and intra-tumour heterogeneity, including the molecular characterisation of multiple samples from individual and successive tumours from each patient taken during disease progression. The studies include both patients with known genetic predisposition and sporadic OC. A long-term goal is to develop biomarkers for early detection and to suggest novel intervention strategies.
Projects
- Multi-level molecular profiling of OC in a heterogeneity context
- Spatiotemporal heterogeneity in high grade serous ovarian cancer (HGSOV)
- Development of prognostic and predictive biomarkers
Selected publications
Sveen A, Johannessen B, Klokkerud SMK, Kraggerud SM, Meza-Zepeda L, Bjørnslett M, Bischof K, Myklebost O, Tasken K, Skotheim RI, Dørum A, Davidson B, and Lothe RA (2024). Evolutionary mode and timing of dissemination of high-grade serous carcinomas. JCI Insight 9(3): e170423
Smebye ML, Agostini A, Johannessen B, Thorsen J, Davidson B, Tropé CG, Heim S, Skotheim RI, and Micci F (2017). Involvement of DPP9 in gene fusions in serous ovarian carcinoma. BMC Cancer 17(1): 642
Smebye ML, Sveen A, Haugom L, Davidson B, Tropé CG, Lothe RA, Heim S, Skotheim RI, and Micci F (2014). Chromosome 19 rearrangements in ovarian carcinomas: Zinc finger genes are particularly targeted. Genes Chromosomes Cancer 53(7): 558-67
Kraggerud SM, Hoei-Hansen CE, Alagaratnam S, Skotheim RI, Abeler VM, Rajpert-De Meyts E, and Lothe RA (2013). Molecular characteristics of malignant ovarian germ cell tumors and comparison with testicular counterparts: Implications for pathogenesis. Endocrine Reviews 34(3): 339-76
Micci F, Skotheim RI, Haugom L, Weimer J, Eibak AM, Abeler VM, Trope CG, Arnold N, Lothe RA, and Heim S (2010). Array-CGH analysis of microdissected chromosome 19 markers in ovarian carcinoma identifies candidate target genes. Genes Chromosomes Cancer 49(11): 1046-53
Micci F, Weimer J, Haugom L, Skotheim RI, Grunewald R, Abeler VM, Silins I, Lothe RA, Trope CG, Arnold N, and Heim S (2009). Reverse painting of microdissected chromosome 19 markers in ovarian carcinoma identifies a complex rearrangement map. Genes Chromosomes Cancer 48(2): 184-93
Hoei-Hansen CE, Kraggerud SM, Abeler VM, Kaern J, Rajpert-De Meyts E, and Lothe RA (2007). Ovarian dysgerminomas are characterised by frequent KIT mutations and abundant expression of pluripotency markers. Mol. Cancer 6: 12
Kraggerud SM, Szymanska J, Abeler VM, Kaern J, Eknaes M, Heim S, Teixeira MR, Tropé CG, Peltomäki P, and Lothe RA (2000). DNA copy number changes in malignant ovarian germ cell tumors. Cancer Res. 60(11): 3025-30