Marte Sneeggen identifies new mechanism how a tumor suppressor prevents metastasis

First author
In a recent article in Nature Communications, published online on the 28thof June, 2019, PhD student Marte Sneeggen and her coworkers at the Institute for Cancer Research and the Center for Cancer Cell Reprogramming (CanCell) identify a new mechanism how the tumor suppressor WDFY2 prevents metastasis by controlling recycling of the matrix metalloproteinase MT1-MMP
One of the most serious complications of cancer is the ability of tumors to form metastases. In most cases, the primary tumor can be treated, whereas metastasis is still the major cause of cancer mortality.
During metastasis, cells migrate and invade into the surrounding extracellular matrix. This is a dense barrier, and cells must actively degrade and remodel this matrix in order to invade and form metastases.
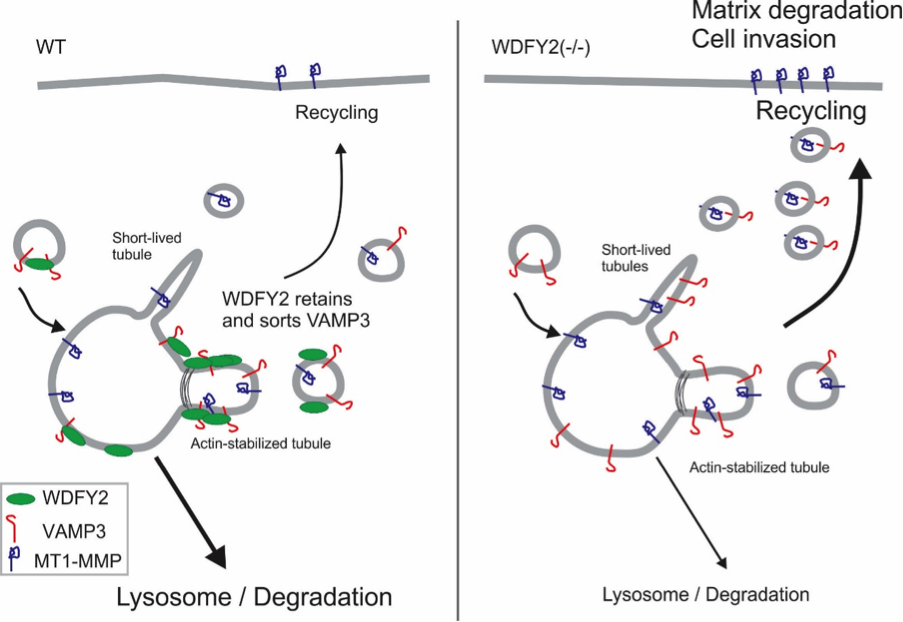
Degradation of the extracellular matrix is performed by matrix metalloproteinases, which digest the matrix material and thereby open a path for the cells to invade. Sneeggen and her co-workers found that an endosomal protein, WDFY2, is critical to regulate the recycling of the matrix metalloproteinase MT1-MMP from endosomes to the cell surface. In normal cells, MT1-MMP recycling is tightly regulated, and WDFY2 slows down the transport of MT1-MMP to the cell surface by sorting it into specific endosomal tubules.
WDFY2 is frequently lost in cancers, especially in high grade ovarian and prostate cancers. If WDFY2 is lost – an event that often happens in metastatic cancers - cells lose control over the recycling of MT1-MMP. More MT1-MMP is transported to the cell surface and allows normally non-invasive cells to become invasive. Using CRISPR/Cas9, Sneeggen et al showed that deletion of WDFY2 in normal cells is sufficient to allow these cells to become invasive, whereas overexpression of WDFY is able to “re-program” highly invasive prostate cancer cells to a non-invasive phenotype. This shows that WDFY2 is a key factor regulating tumor cell invasion and metastasis.
In the future, it will be interesting to address if loss of WDFY2 is a diagnostic biomarker for metastasis and can be used to guide treatment.
The article, published 28 June 2019:
WDFY2 restrains matrix metalloproteinase secretion and cell invasion by controlling VAMP3-dependent recycling
Marte Sneeggen, Nina Marie Pedersen, Coen Campsteijn, Ellen Margrethe Haugsten, Harald Stenmark & Kay Oliver Schink
Nature Communications, Volume 10, Article number: 2850 (2019)
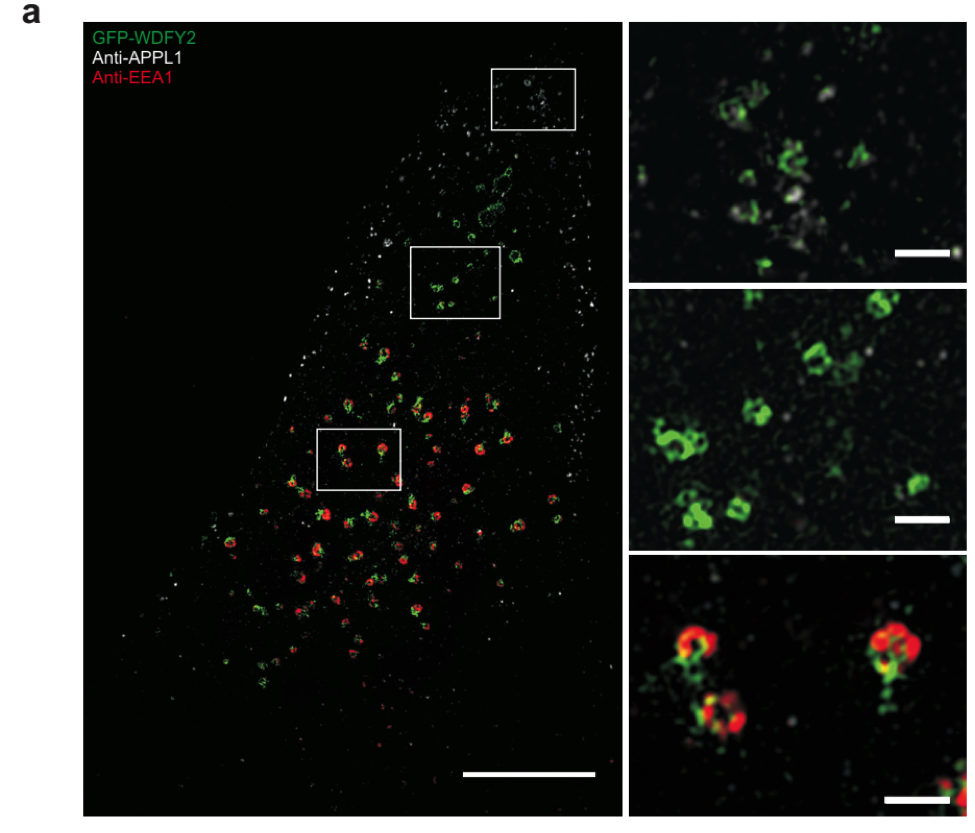
Superresolution image of WDFY2 localization. WDFY2 localizes to tubular structures on endosomes.
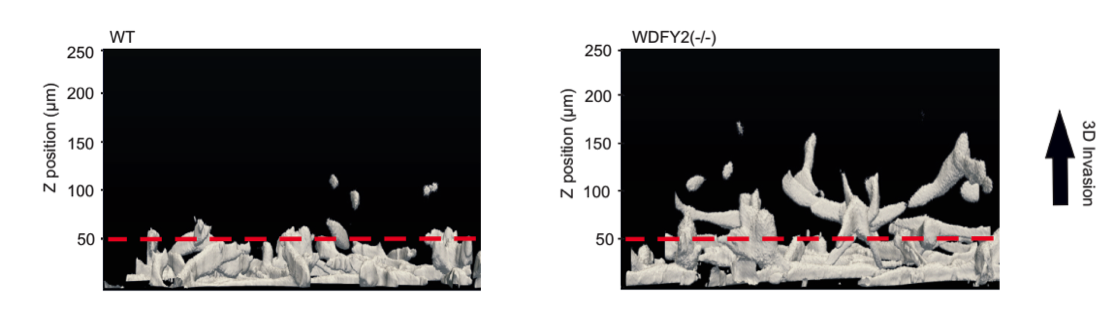
Deletion of WDFY2 by CRISPR/Cas9 leads to enhanced cell invasion.
Links:
Home pages:
Harald Stenmark's Cellular membrane dynamics group
Kay Oliver Schink's Phosphoinositide control of early endocytic trafficking project group
