Raiborg and Stenmark with review article in Nature

In the March 26th issue of Nature, Camilla Raiborg and Harald Stenmark at the Centre for Cancer Biomedicine, Institute for Cancer Research, have published a review about the functions of endosomal sorting complex required for transport (ESCRT) proteins in endosomal sorting of ubiquitylated membrane proteins.
In the review, the authors disucss that selective trafficking of membrane proteins to lysosomes is required for proper cell signalling and metabolism. Ubiquitylation signals this by specifying protein transport to the lysosome lumen via the multivesicular endosome pathway. The ESCRT machinery sorts ubiquitylated cargo into invaginations of endosome membranes and, through a highly conserved mechanism also employed in cytokinesis and viral budding, mediates abscission of the cargo-containing intraluminal vesicles from the perimeter membrane. The involvement of the ESCRT machinery in suppressing diseases such as cancer, neurodegeneration and infections underscores its importance in cell biology and physiology.
Figure: The ESCRT machinery in endosomal sorting of ubiquitinated membrane proteins.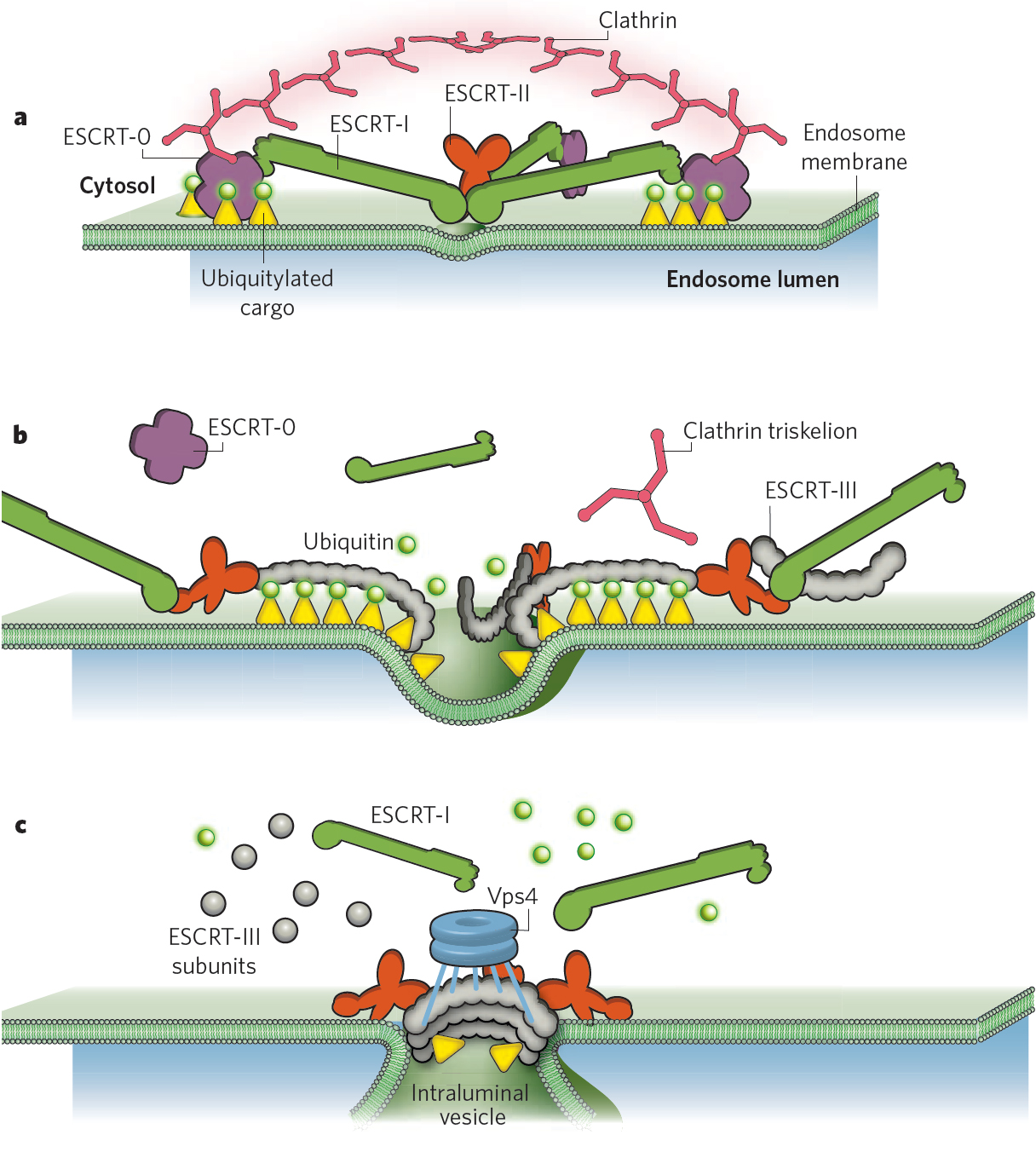
b) Membrane deformation. ESCRT-III is recruited by binding to the two Vps25 subunits of ESCRT-II and forms spiral-shaped filaments that gate cargo into invaginations that are caused by the ESCRT-III filaments. During this process, cargo is deubiquitinated by proteases that are recruited by ESCRT-III, but the diffusion of cargo is strictly limited by the ESCRT-III filaments.
c) Membrane abscission. As ESCRT-III filaments assemble into circular arrays, the membrane continues to invaginate. Vps4 enters into the invagination to disassemble ESCRT-III filaments, ensuring that filaments only assemble at the neck of the forming ILV. Vps4 may also serve to remove subunits of the neck filaments,thereby contributing to construction of the neck which ultimately causes membrane abscission.
