Genomic instability as a driver of ageing
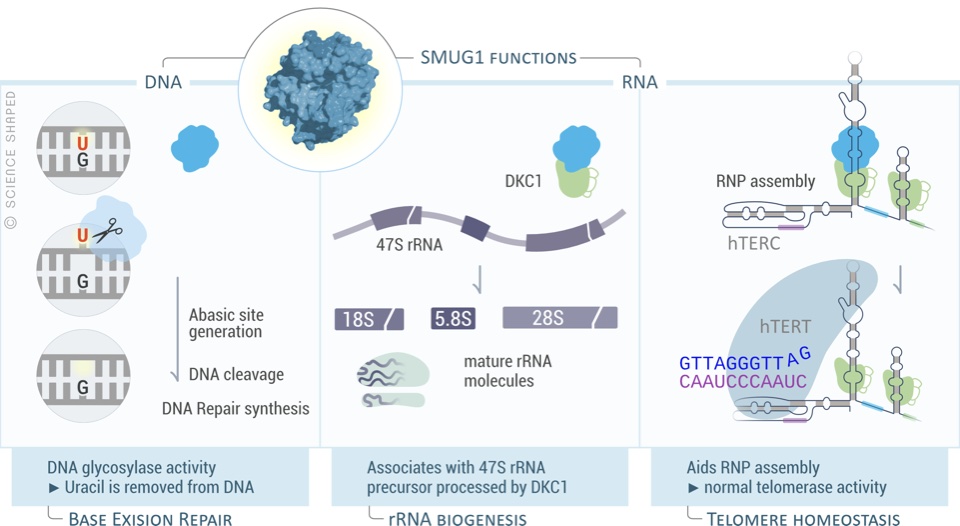
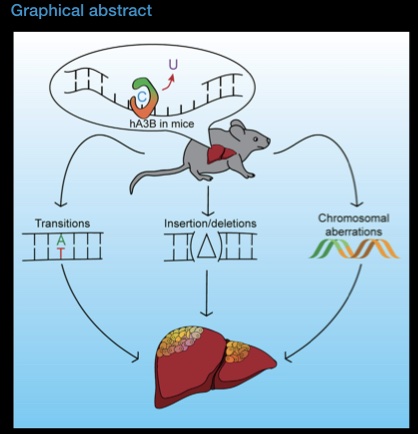
DNA damage is a driver of aging. We study how ageing leads to a loss of coordination within the Base Excision repair pathway, which is essential for maintenance of genomic integrity in mitochondria and nuclear DNA.
In ageing neurons, loss of coordination of Base Excision Repair leads to accumulation of half-repaired DNA molecules which might be more harmful than the original damage. We study the mechanisms of how Base Excision Repair in the nucleus and mitochondria affect aging neurons. We are also interested in how DNA repair can be modulated (pharmacologically and genetically) to promote healthy aging.
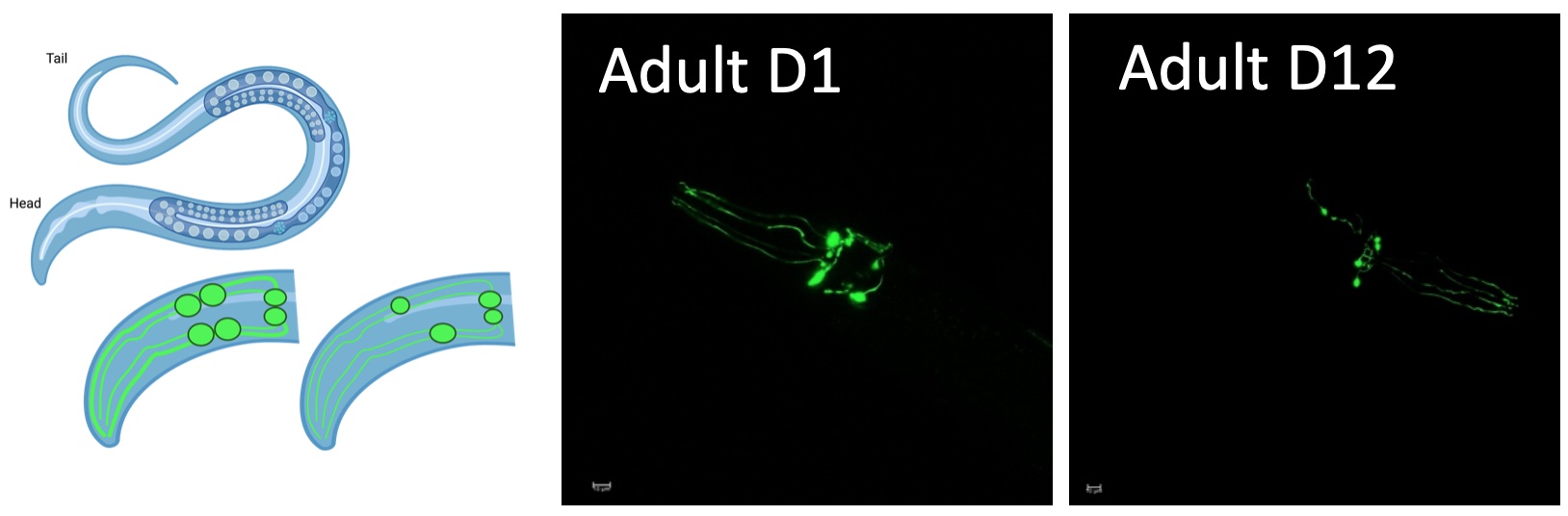
We use C. elegans as a model to study DNA repair across the lifespan. C. elegans expressing human alpha synuclein can be used to study how dopaminergic neurons are kept healthy during aging. Photo Fransisco Naranjo https://doi.org/10.1016/j.celrep.2021.109668
Link to popular scientific description in Norwegian and English: