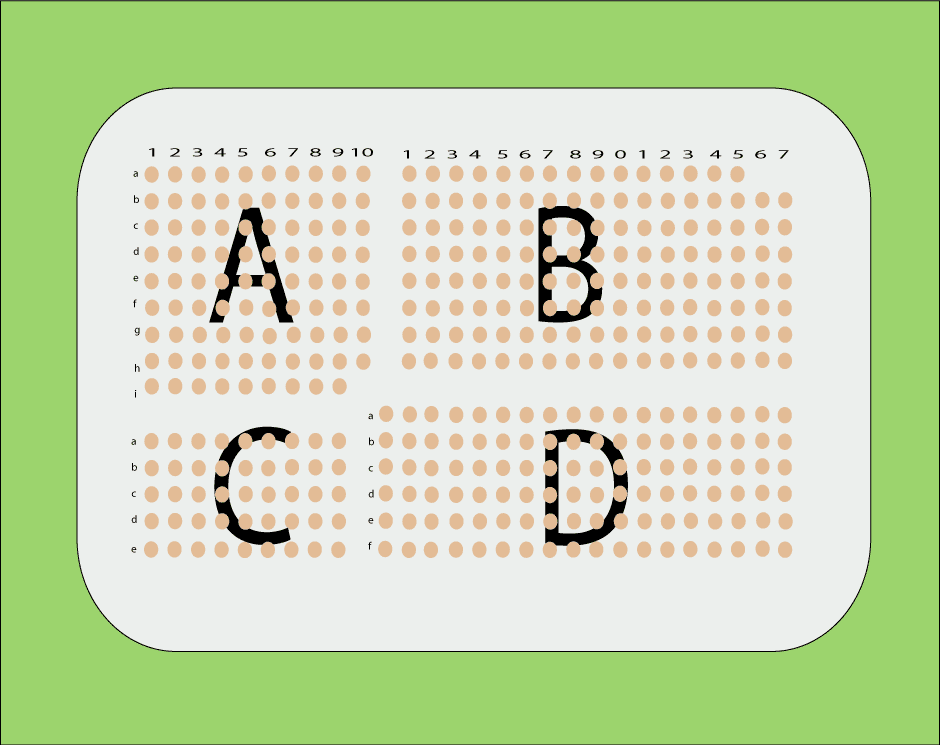
Tissue array
History
Scientific work using this method was first published in 1998 (Kononen et al, 1998). In our lab. we started using tissue micro array in 2001. At present we have three internal projects in progress using tissue micro array. In addition we also cooperate with different other laboratories on scanning and scoring of arrays.
Description
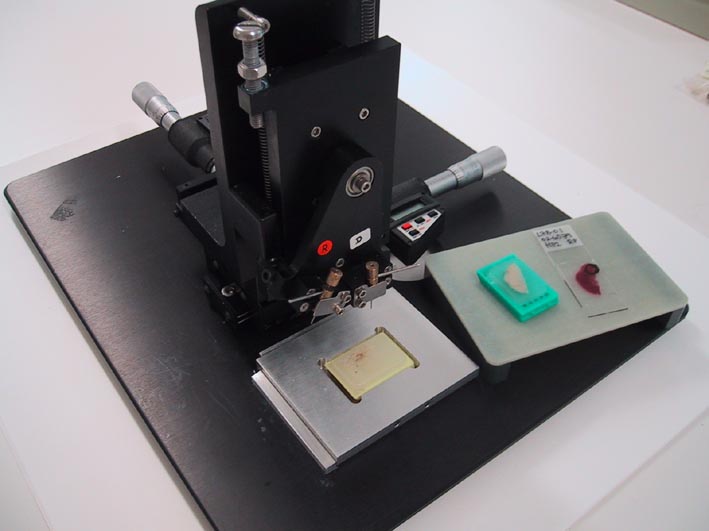
The sampling of the original tissues for arraying is performed from morphologically representative regions of regular formalin-fixed paraffin-embedded tumour blocks. Analysis of a haematoxylin-eosin-section is necessary to localize the area of interest. Core tissue biopsies (diameter 0,6 mm, height 3-4 mm) are taken from individual donor blocks and arrayed into a new recipient paraffin block (35 x 23 mm) using a tissue micro arraying instrument (Beecher Instruments). The recipient block should not have any cracks or damage of any kind, and the paraffine surface should be cut plane using a microtome. We use 0,6 mm cylinders, and 0,4 mm spacing between cylinders. The donor block is manually positioned for sampling based on a visual alignment with the corresponding HE-stained section on a slide.
Scientific work using this method was first published in 1998 (Kononen et al, 1998). In our lab. we started using tissue micro array in 2001. At present we have three internal projects in progress using tissue micro array. In addition we also cooperate with different other laboratories on scanning and scoring of arrays.
Description
The sampling of the original tissues for arraying is performed from morphologically representative regions of regular formalin-fixed paraffin-embedded tumour blocks. Analysis of a haematoxylin-eosin-section is necessary to localize the area of interest. Core tissue biopsies (diameter 0,6 mm, height 3-4 mm) are taken from individual donor blocks and arrayed into a new recipient paraffin block (35 x 23 mm) using a tissue micro arraying instrument (Beecher Instruments). The recipient block should not have any cracks or damage of any kind, and the paraffine surface should be cut plane using a microtome. We use 0,6 mm cylinders, and 0,4 mm spacing between cylinders. The donor block is manually positioned for sampling based on a visual alignment with the corresponding HE-stained section on a slide.