Childhood cancer
Projects:
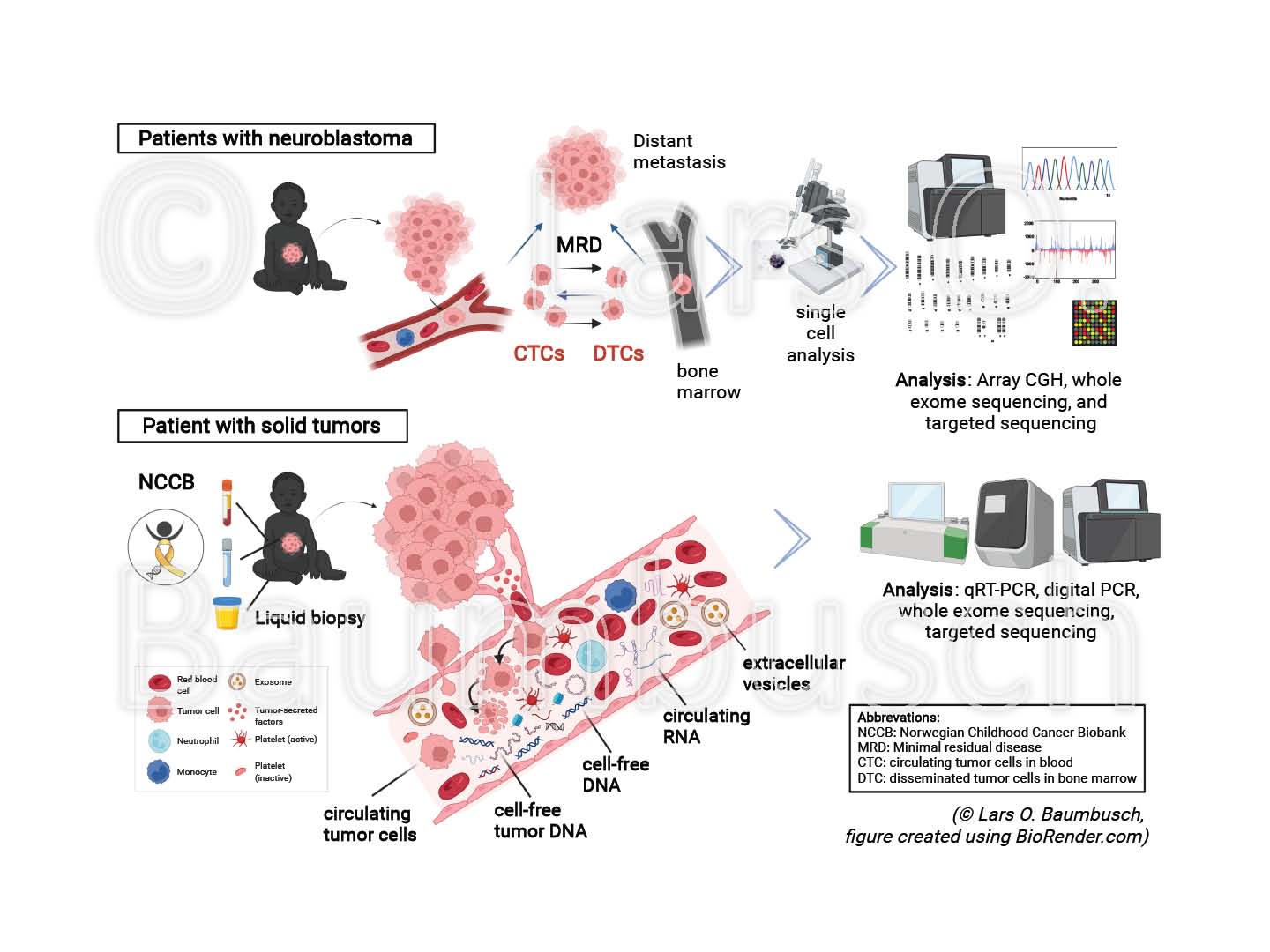
Exploring liquid biopsies in pediatric cancer - promising targets for clinical benefits
Childhood cancers remain the dominant cause of death by disease among children over one year in developed countries. Biopsy of a tumor is the principal object for cancer diagnostics today; however, unsatisfactory due to limitations in the access, quality or amount of the collected material.
Liquid biopsy is a novel, promising tool for diagnosis and treatment, with reduced invasiveness for the patient. The technique is based on the knowledge that solid tumors release circulating tumor cells (CTCs), cell-free tumor DNA (ctDNA), circulating RNA (circRNA), extracellular vesicles (EVs) and further molecular structures into the blood stream and other body fluids. Liquid biopsy provides a more authentic and comprehensive picture of the mutational load, improving early detection, stratification and monitoring of potential disease recurrence.
We have characterized previously disseminated tumor cells using array CGH and sequencing technology, analyzed cfDNA from various body fluids and examined non-coding RNA using qRT-PCR and droplet digital PCR (ddPCR) in model organisms and cancer patients.
In this project, we will investigate samples from pediatric patients with solid tumors collected in the Norwegian Childhood Cancer Biobank (NCCB). It is planned to explore the characteristics and changes of ctDNA, circRNA, EVs and exosomes, in relation to each other and to the status of the tumor at diagnosis and relapse to reveal their implication to the onset of cancer. To this end, we will test and establish advanced liquid biopsy methods including ddPCR and sequencing technology. We believe that understanding these novel groups of circulating molecular structures will pave the way or changing the current clinical practice and knowledge in the future.
PhD student: Benedicte Grebstad Tune.
This project is funded by the Barnekreftforeningen.
This project resulted in the following publications:
Tune BG, Sareen H, Powter B, Kahana-Edwin S, Cooper A, Koh E-S, Lee CS, Po JW, McCowage G, Dexter M, Cain L, O’Neill G, Prior V, Karpelowsky J, Tsoli M, Baumbusch LO, Ziegler D, Roberts TL, DeSouza P, Becker TM, and Ma Y (2023) From pediatric to adult brain cancer: exploring histone H3 mutations in Australian brain cancer patients. Biomedicines 11, 2907. doi.org/10.3390/biomedicines11112907.
Investigation of the microbiome in childhood cancer
Every year about 180-200 children develop cancer in Norway. Child cancer is still the predominant cause of death among children over a year in Western countries despite of significantly improved treatment and increased knowledge of the molecular mechanisms of various cancers. Unfortunately, there are defined groups of childhood cancer characterized by short survival, unexpected relapses and/ or lifelong side effects. Identification of previously unrecognized mechanisms driving carcinogenesis would help develop robust biomarkers, which are urgently needed for the diagnosis and therapeutic management of childhood cancer. The human microbiota plays an important role in health and disease. The microbial community of the human gastrointestinal tract, the gut microbiome, has been implicated as a possible driver and regulator of cancer pathogenesis. Several studies have reported associations between microbial alterations and different types of cancers; however, the gut microbiome has not been systematically characterized in childhood cancer. This project will increase understanding of the possible importance of the microbial in the development, progression and/or and tolerance and effect of treatment in childhood cancer. Sequencing of 16s rRNA using MiSeq in stools from children with newly diagnosed childhood cancer, with procedure established previously by our collaboration partners. By using DNA sequencing methods and metabolite profiling, we will characterize features of the gut microbiome that associate with different cancer diagnoses in children. This could contribute to a more targeted strategy for cancer treatment in the near future.
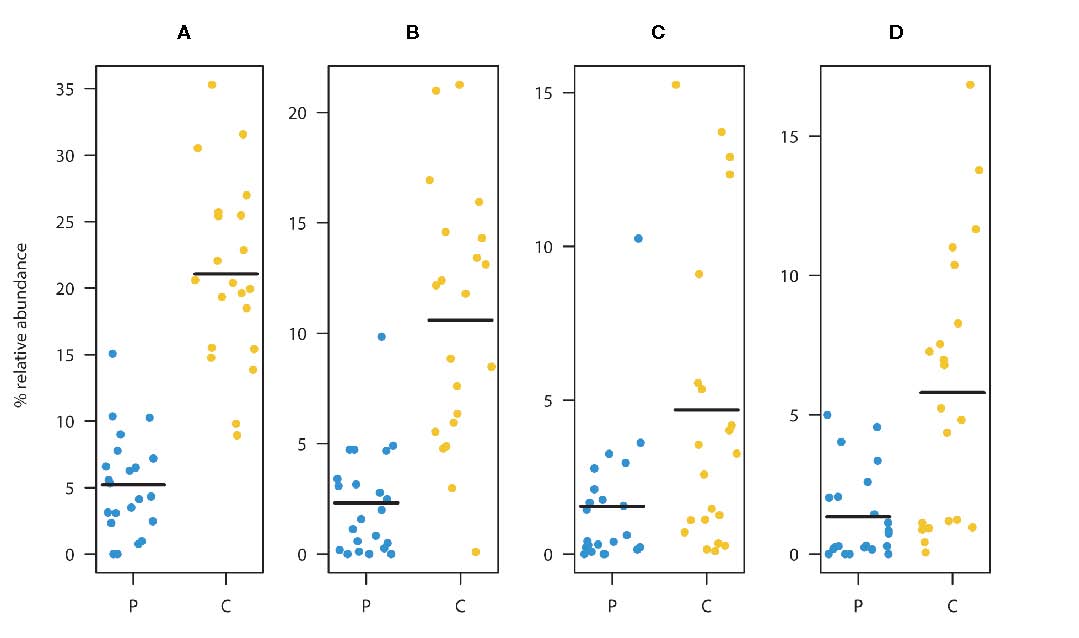
Faecalibacterium prausnitzii (A). Summed relative abundances of ASV2, ASV3, and ASV6
(main taxa assigned to F. prausnitzii). Relative abundances of ASV2 (B), ASV3 (C), and ASV6 (D).
These ASVs were classified as F. prausnitzii and were found to be significantly depleted in the patient group
relative to the healthy controls. (Black lines represent means, yellow dots represent the individual relative
abundances of the controls three years of age and older, and blue dots represent the individual relative
abundances in the children three years of age and older in the diagnosed group).
(From: de Muinck et al., https://doi.org/10.3389/frmbi.2023.1151889; © 2023 by the authors).
The project is in close collaboration project with Veronika Kucharová Pettersen at UNN.
This project involves the NCCB and is funded by the Barnekreftforeningen.
This project resulted in the following publications:
de Muinck EJ, Trosvik P, Nguyen N, Fashing PJ, Stigum VM, Robinson N, Hermansen JU, Munthe-Kaas MC, and Baumbusch LO (2023) Reduced abundance of Faecalibacterium prausnitzii in the gut microbiota of children diagnosed with cancer, a pilot study. Frontiers in Microbiomes 2:1151889. doi.org/10.3389/frmbi.2023.1151889.
Exploring the heterogeneity of neuroblastoma by single cell technologies
We aim to improve the life expectancy of children with pediatric cancer, as childhood malignancies are still the dominant cause of death by disease among children over one year of age in high income countries. Heterogeneity represents one of the fundamental challenges for cancer diagnosis and treatment. We hypothesize that investigating the heterogeneity of the primary tumor and minimal residual disease at the genomic, transcriptomic, and proteomic level will lead to an enhanced understanding of tumor aggressiveness and therapy response.
Neuroblastoma (NBL) is a cancer of the early childhood, characterized by extreme clinical, biological, and genetic diversity. High-risk NB patients with primary resistant or relapsing disease are treated with one of the most intensive therapy regiments in modern pediatric oncology; nevertheless, a successful treatment is currently missing and the prognosis is poor.
We will explore the primary tumor and corresponding disseminated tumor cells applying single cell methods in combination with sequencing technologies, established from adult cancers and supported by advanced bioinformatic tools. Initial findings will be verified in a larger validation cohort and by functional cell-lines studies. We believe that understanding the genetic and compositional heterogeneity in tumors is crucial for essential progress of improved drug development, biomarkers, and treatment susceptibility.
PhD student: Kirsti Marie Gjersvoll Paulsen.
This project involves the NCCB. and is funded by the Barnekreftforeningen and the Jonathans Minnefond.
This project resulted in the following publications:
Przybyła W, Paulsen KMG, Mishra CK, Nygård S, Engebretsen S, Ruud E, Trøen G, Beiske K, and Baumbusch LO (2022) Whole exome sequencing of high-risk neuroblastoma identifies novel non-synonymous variants. PLoS ONE 17(8):e0273280. doi.org/10.1371/journal.pone.0273280.
Exploring high-risk neuroblastoma by sequencing technology
Next generation sequencing will be the basis for personalized cancer medicine in the near future, in particular for heterogenic diseases with multi-modal therapy applications like neuroblastoma (NBL) - one of the major challenges in pediatric oncology. We hypothesize that comparative sequencing analysis will provide means about heterogeneity and relapse in high-risk NB. Patient samples are available from an existing diagnostic biobank and novel patients will be enrolled in a Norwegian pediatric cancer sequencing biobank project. In collaboration with our scientific partners, the required technologies are in place, like aCGH and single cell aCGH, sequencing techniques, further comparative analysis of high resolution data. We are convinced that investigating the genome and transcriptome of the primary tumor, relapse tissue, and micrometastases in high-risk NB will reveal novel insight into the heterogeneity, tumor evolution, and biology of NB - a promising prospective for new therapies for children fighting NBL.
PhD student: Weronika Przybyła.
This project is funded by the South-Eastern Norway Regional Health Authority.
This project resulted in the following publications:
Przybyła W, Paulsen KMG, Mishra CK, Nygård S, Engebretsen S, Ruud E, Trøen G, Beiske K, and Baumbusch LO (2022) Whole exome sequencing of high-risk neuroblastoma identifies novel non-synonymous variants. PLoS ONE 17(8):e0273280. doi.org/10.1371/journal.pone.0273280.
 The Norwegian Childhood Cancer Biobank
The Norwegian Childhood Cancer Biobank
Pediatric cancer is the dominant cause of death in children over one year of age in western countries. New treatment strategies are warranted, and rapid advancements in molecular and genetic sequencing technology are realizing the potential for improving care with precision medicine. In pediatric oncology, this encompasses identification of unique cancer predispositions, somatic tumor mutations, personalized surveillance, and targeted treatment. However, the clinical benefits of these scientific advancements in the future rely on the development of translational bioinformatic expertise, clinical pipelines for extended testing and services, and ethically sound information and decision systems for families and health workers within pediatric oncology. Our ultimate goal is to use the generated knowledge towards the establishment of a general translational platform including different molecular modalities, where novel molecular and genetic information is systematically translated into clinical application.
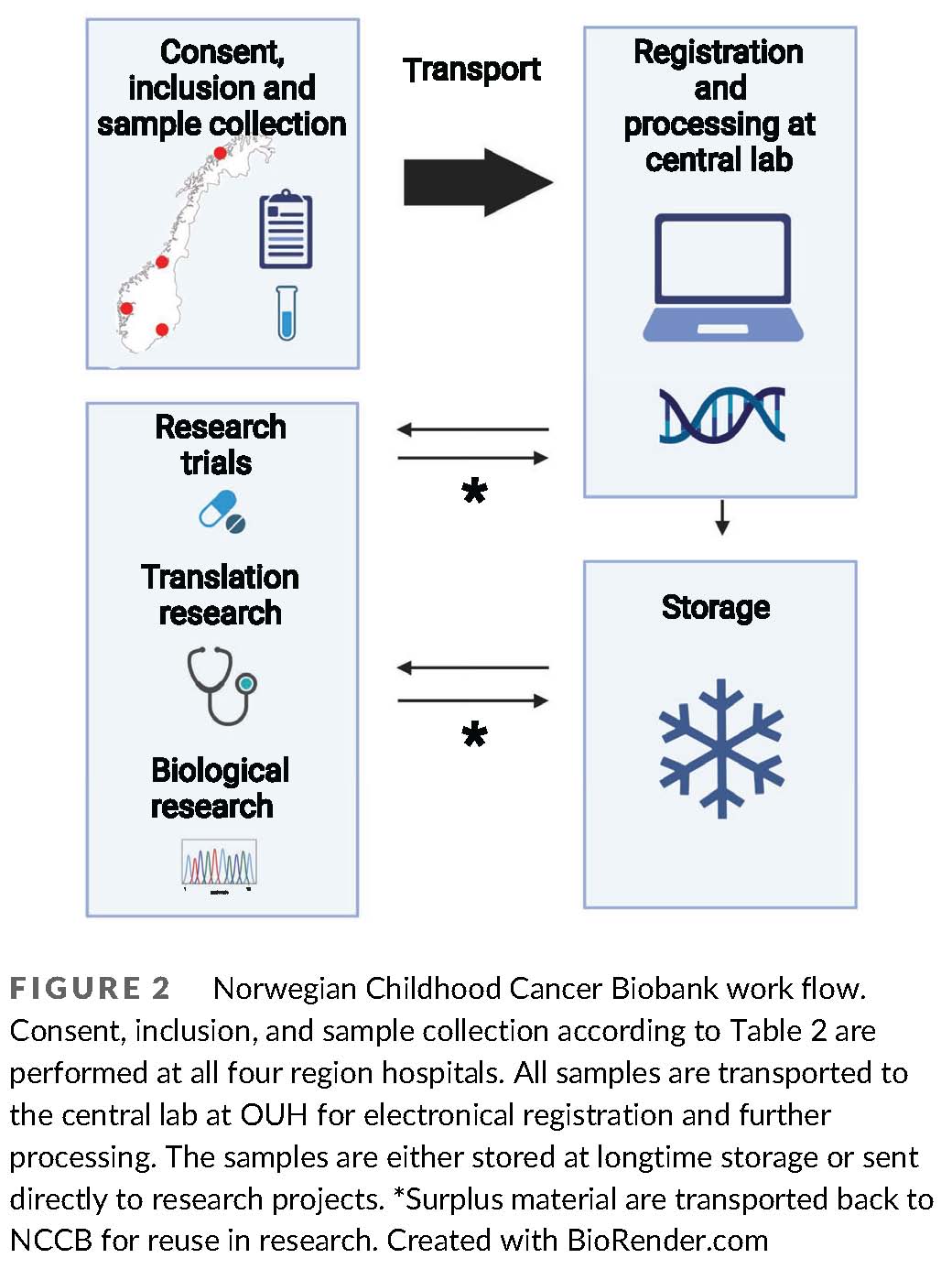
(From: Hermansen et al., https://doi.org/doi.org/10.1002/cnr2.1555;
© 2021 by the authors).
Link to the Norwegian Childhood Cancer Biobank
This project is supported by the Barnekreftforeningen
This project resulted in the following publications:
Hermansen JU, Wojcik DM, Robinsin N, Pahke J, Haugland HK, Jamtøy AH, Flægstad T, Halvorsen H, Lund B, Baumbusch LO‡, and Munthe-Kaas MC‡ (2021) The Norwegian Childhood Cancer Biobank. Cancer Reports 5(8):e1555. doi.org/10.1002/cnr2.1555.
(‡The marked authors should be considered as joint senior authors)
Supported by ![]()
![]()
![]()
