NorMa: Normal mammary tissue and cancer development
NorMa – Normal Mammary tissue
Main objective:
Molecular studies on breast tissue from healthy women, with varying risk of breast cancer development.
Aim of study:
The study is defined in two set of aims; short-term aims and long-term aims.
1. Short-term aims:
- Reveal the differentiation route of epithelial cells
- Establish reproducible self-organizing mammary organoid cultures from single cells derived from healthy women
- Explore the pattern of development of different breast cancer sub-types
- Detect how the micro environment affect the differentiation of epithelial cells and cancer sub-type development
2. Long-term aims:
- Reveal the molecular profiles of normal breast tissue associated with increased risk of breast cancer development
- Achieve increased knowledge of breast cancer initiation and progression
Objects of investigation:
Three groups of women is investigated
- Group 1. Low-risk: Reduction mammoplastics
- Group 2. Increased-risk: Benign lesions
- Group 3. High-risk: a) Breast cancer patients (Ipsilateral, contralateral) b) Profylactic mastectomies
Collected materials:
- Healthy breast tissue for isolation of viable cells/ organoids
- Healthy breast tissue (fresh-frozen)
- Tumor tissue (fresh-frozen)
- Biopsy from healthy tissue (contralateral)
- Blood samples (EDTA, plasma, serum)
Establishing mammary organoid cultures from single cells derived from healthy women
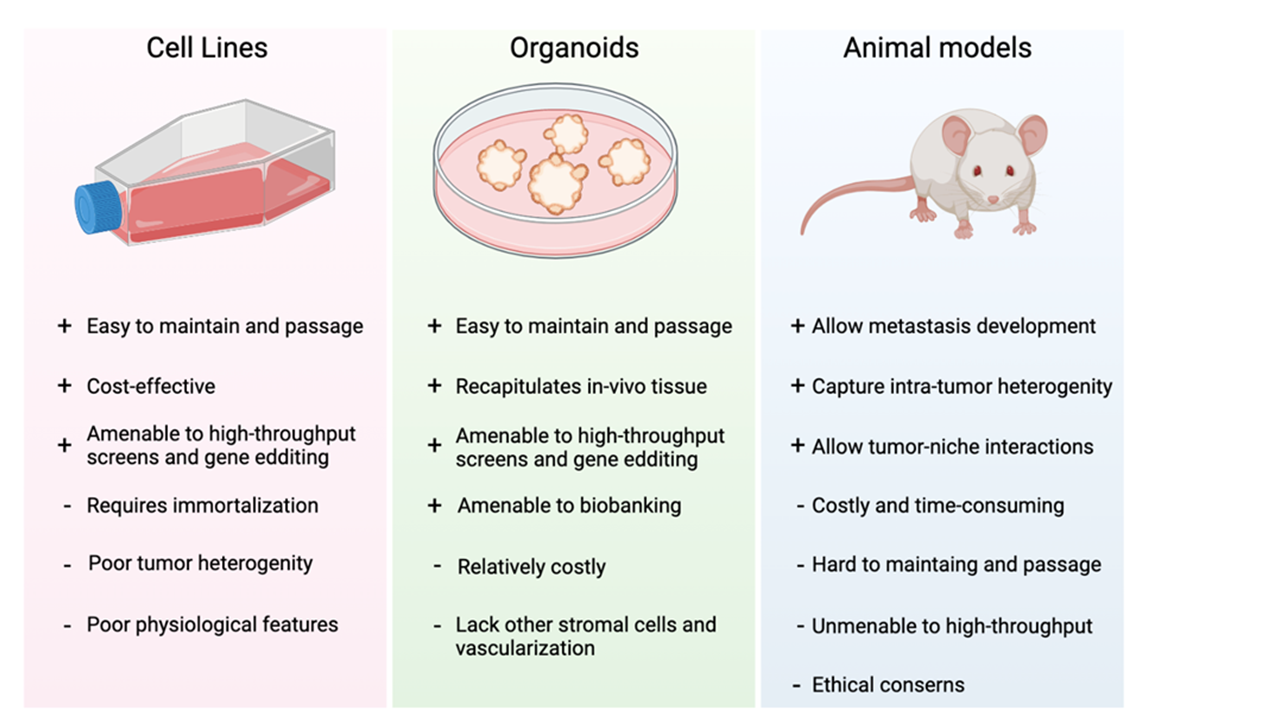
Cell lines (in vitro)
- Human immortalized cells that divide as long as they have access to space and media
- Two dimensional cells that are prone to genetic drift over time
Organoids
- Complex self-organizing three-dimensional structures that presents a bridge between cell lines and animal models
- Recapitulates the human tissue in a more physiological way
- Amenable to biobanking
Animal models (in vivo)
- Great humanized models that allow the study of tumorigenesis from a patients tumor or the study of gene function
Methods:
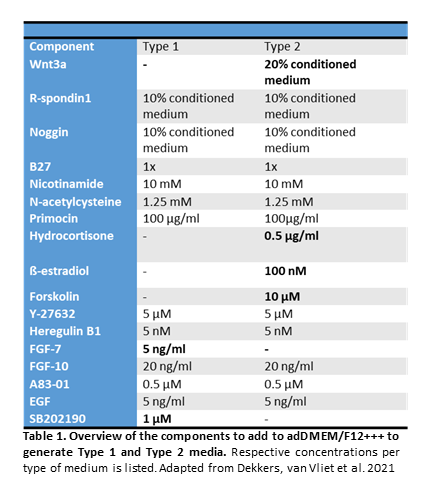
- Adapted a protocol from Dekkers et. al. (2021) for organoid establishment
- Using two types of expansion media (Type 1 and Type 2)
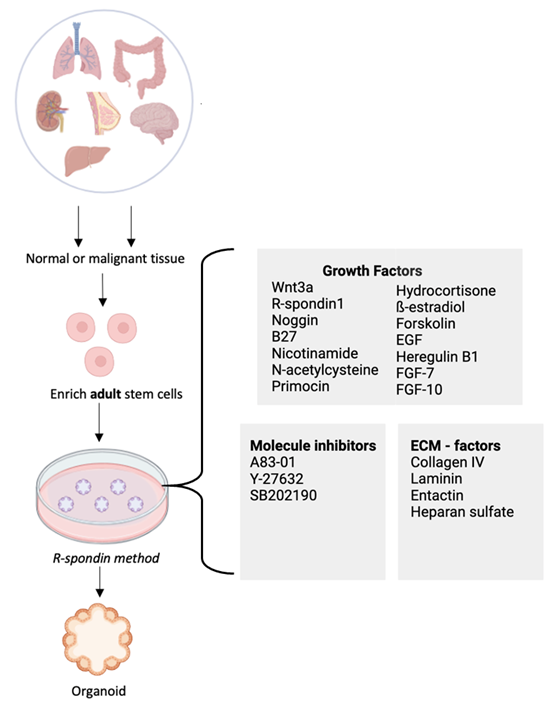
Three-dimensional organoid cultures are a relatively new in vitro model technology that can recapitulate the in vivo tissue with a similar architecture and physiology than other systems like cell lines and animal models. Culturing adult stem or progenitor cells derived from the tissue of an individual, with the support of an extracellular matrix and a cocktail of growth factors can maintain the stem cell niche and the epithelial hierarchy and allow the generation of a miniature organ in a dish. These organoid cultures can enable studies like organ development, onset of disease, and personalized medicine in patients.
Results: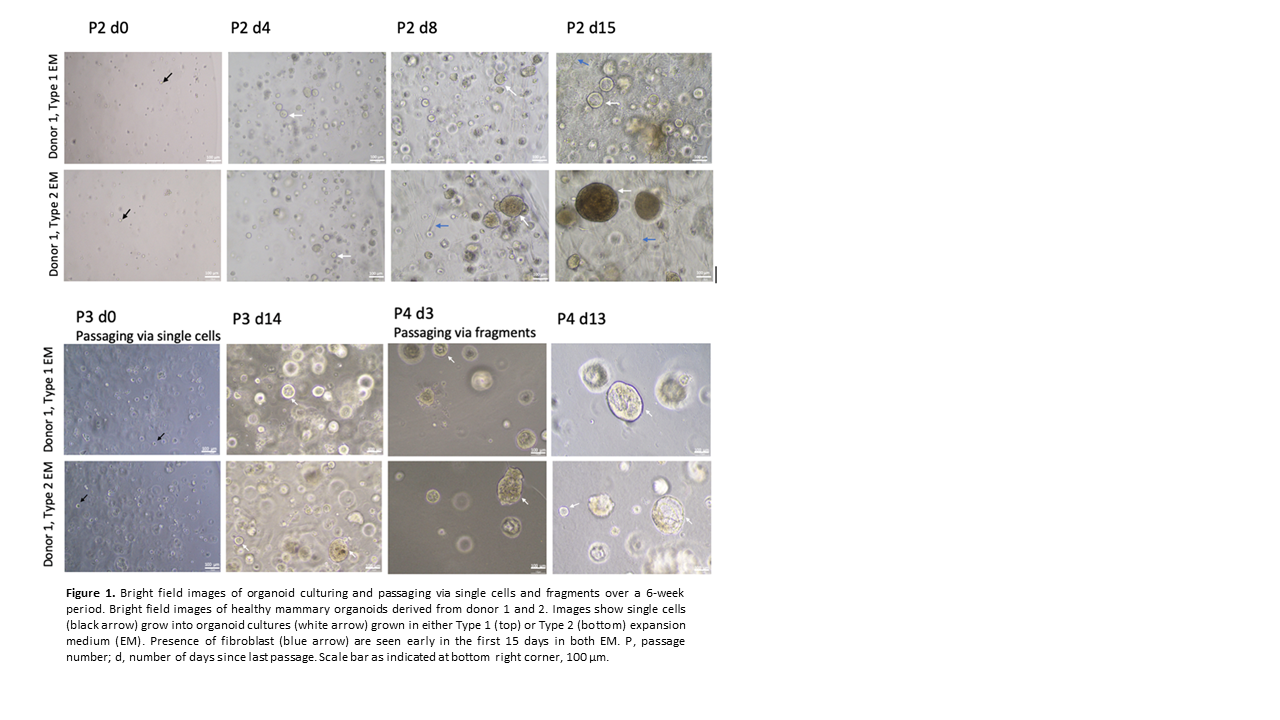
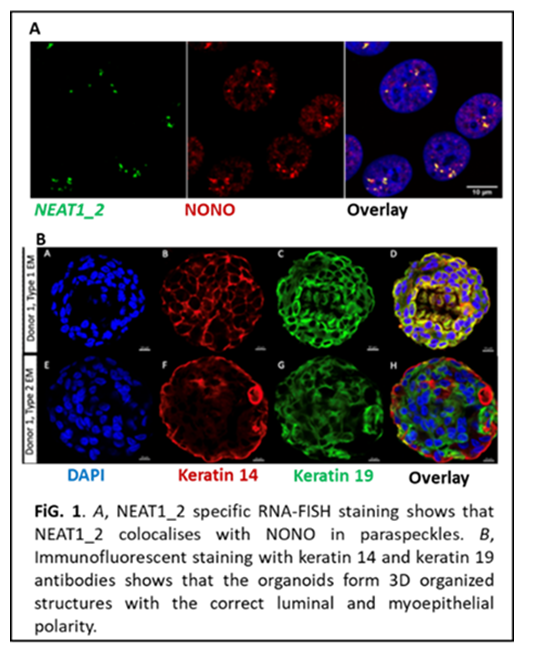
Expression of NEAT1 isoforms in mammary organoid cultures
Aim
The aim of this project is to provide novel insights into expression and functions of breast cancer associated NEAT1 in mammary epithelial cells by determining the expression of NEAT1_1 and NEAT1_2 isoforms in mammary organoid cultures.
Background and approach
Nuclear paraspeckle assembly transcript 1 (NEAT1) is a highly abundant nuclear long non-coding RNA. There are two overlapping NEAT1 isoforms, NEAT1_1 and NEAT1_2, of which the latter is an essential scaffold for the assembly of nuclear bodies called paraspeckles. Paraspeckle formation is elevated upon cellular stress and at certain developmental stages where they modulate cellular processes by molecular sequestration. NEAT1 has an important role in postnatal mammary gland development in mice and is abnormally expressed in many breast cancers as well as other cancers. Importantly, high NEAT1 levels are associated with therapy resistance and poor clinical outcome. In breast cancer, the relative expression of NEAT1_1 and NEAT1_2 appears to be different among the intrinsic subtypes, which might reflect distinct expression pattern of the two isoforms in different subpopulations of mammary epithelial cells. Although not studied in mammary cells, NEAT1_1 is the dominating isoform in stem cells whereas a switch from NEAT1_1 to NEAT1_2 expression occurs during differentiation. To provide novel insights into NEAT1 isoform expression in mammary cells of different differential states (luminal epithelial and myoepithelial lineages, stem cells, unipotent progenitors), we will determine the expression of NEAT1_2 and total NEAT1 (NEAT1_1 + NEAT1_2) in mammary organoid cultures derived from healthy women (reduction mammoplasties). We will use a well-established RNA-fluorescent in-situ hybridization protocol (UiT) (Fig. 1A) and a robust and highly reproducible protocol of 3D organotypic cultures of primary cells derived from the human breast that form 3D organized structures with the correct luminal and myoepithelial polarity (Department of Medical Genetics, OUH) (Fig 1B). Here, we will conduct a pilot experiment where NEAT1 isoform expression is analysed in 10 organoid cultures from healthy donors.
Future follow up: The pilot experiment will be followed up by expression analyses of NEAT1 isoforms in breast cancer (BC) patient-derived organoid cultures determine the differential expression of the isoforms in BC subtypes."
Project members: David Kunke, Miriam Heggøy, Marie Fongaard, Grethe I. Grenaker Alnæs, Torben luders, Xavier Tekpli
External colaborators:
- Alex Corthay, Rikshospitalet https://www.ous-research.no/corthay/
References: