Radium Hospital researchers with comment in Cell

In the January 9th issue of Cell (impact factor 29.887), Lene Malerød and Harald Stenmark at the Department of Biochemistry, Institute for Cancer Research, highlight recent research that defines the sequential activation, recruitment, and disassembly of ESCRT-III subunits during membrane involution in vitro.
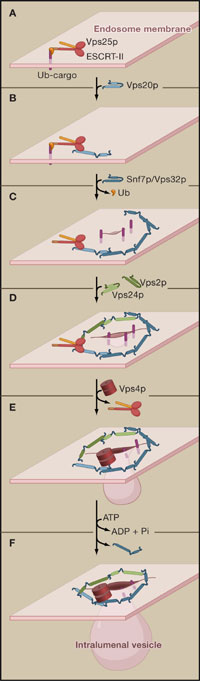
Ordered assembly and disassembly of the ESCRT-III complex to mediate cargo sorting and invagination of endosome membranes.
Assembly starts with recruitment of the ESCRT-III subunit Vps20p by the ESCRT-II subunit Vps25p on endosomes (A), which causes activation of Vps20p through release of an autoinhibitory interaction (B). The activated Vps20p in turn binds Snf7p/Vps32p and nucleates Snf7p monomers to oligomerize into circular filaments that mediate cargo entrapment and membrane invagination (C). Upon binding of Vps24p-Vps2p, oligomerization terminates (D) and Vps4 is recruited (E) and facilitates disassembly of ESCRT-III by releasing Snf7p molecules (F). The Vps4p-mediated removal of Snf7p monomers at the end of the filament might cause its constriction, ultimately leading to membrane abscission.
Each individual interaction facilitates specific conformational changes opening up and closing protein structures allowing only specific partners to interact. Cumulatively, these changes in protein conformations lead to the recruitment of a specific sequence of factors, thereby driving and controlling each step along the pathway for cargo sorting and membrane deformation. ESCRT-III-mediated membrane deformation in cytokinesis and viral budding is likely to be equivalent to that proposed for the biogenesis of intralumenal vesicles, with the exception that the initial recruitment and activation of Vps20 is mediated by other components.
Â
Links:
ESCRTing membrane deformation. (link to PubMed)
Malerød L, Stenmark H.
Cell. 2009 Jan 9;136(1):15-7.