Report in Current Biology by Lie-Jensen and Haglund: Parallels between ALIX recruitment during cytokinetic abscission in flies and virus budding in human cells

Understanding how abscission between daughter cells during the final stage of cell division, cytokinesis, is accurately controlled is relevant for cancer, because failure in this process may give rise to binucleate cells and in the next cell division chromosomal missegregation, which might cause aneuploidy, a common feature of cancer cells. In a recent issue of Current Biology, Lie-Jensen and co-authors elucidate a mechanism by which the abscission machinery is recruited to the midbody in the fruit fly Drosophila melanogaster during abscission.
The highly conserved endosomal sorting complex required for transport (ESCRT)-III forms spiral-like filaments that promote scission of thin membrane stalks during diverse cellular processes, including intraluminal vesicle formation at multivesicular endosomes, virus budding and cytokinetic abscission1-5. The scaffold protein ALIX is a core component of the abscission machinery with an evolutionarily conserved role in midbody recruitment of ESCRT-III6-10.
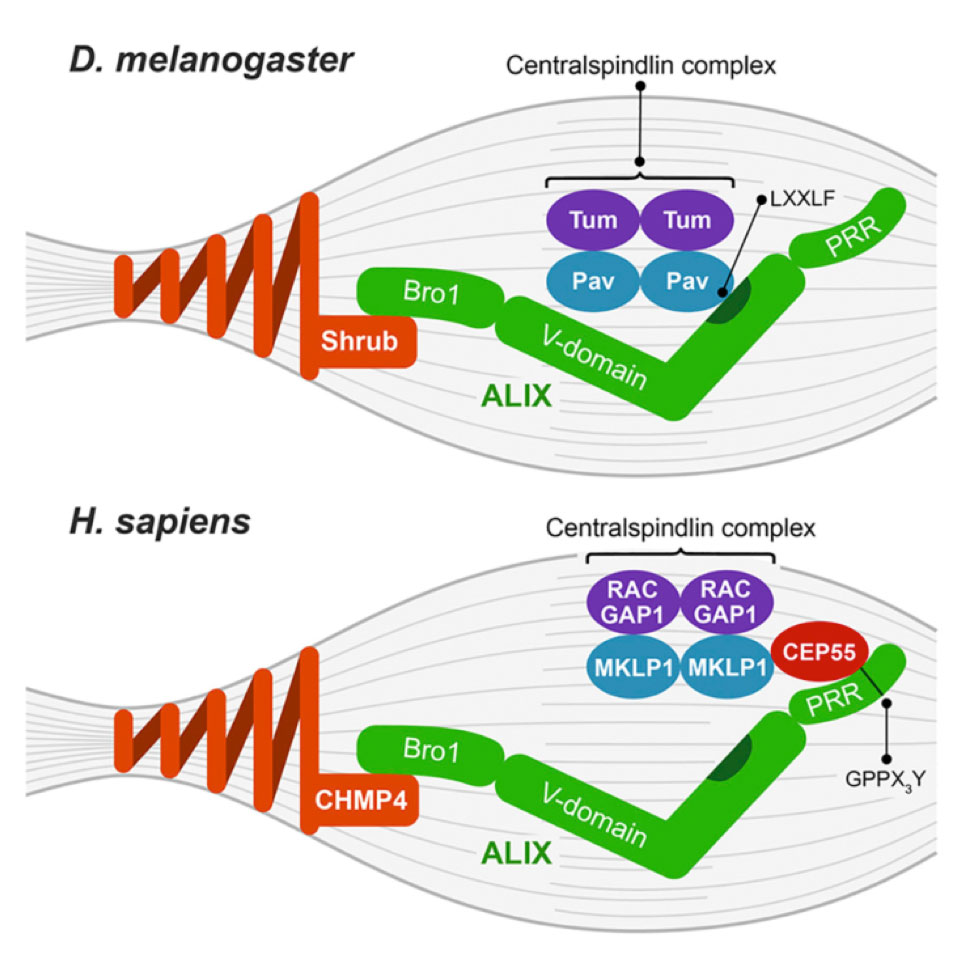
The molecular mechanisms of how key components of the abscission machinery, including ALIX and ESCRT-III, are recruited to the midbody in human cells have been largely elucidated6-8, 11-14, but how the abscission machinery is recruited to the midbody in vivo in Drosophila melanogaster has remained uncharacterized. In the current study, Lie-Jensen et al. show that, during abscission, Drosophila ALIX is recruited to the midbody by a different domain than human ALIX, namely via its V-domain (Figure 1)15. They identify that the centralspindlin subunit Pavarotti interacts with and recruits ALIX to the midbody (Figure 1)15.
During virus budding in human cells, a highly conserved hydrophobic pocket in the ALIX-V-domain interacts with LYPXnL/LXXLF motifs of virus proteins16-18. Virus proteins in this way recruit ALIX, which in turn promotes ESCRT-III recruitment to the virus budding site16-18. Interestingly, the authors elucidate that the conserved hydrophobic pocket in the ALIX-V-domain interacts with Pavarotti and mediates midbody recruitment of Drosophila ALIX (Figure 1)15. At the molecular level they uncover that Pavarotti binds the conserved hydrophobic pocket in the ALIX-V-domain via an LXXLF motif, thereby recruiting ALIX to the midbody and promoting abscission (Figure 1)15. The study thus identifies that ALIX is recruited to the midbody during cytokinetic abscission in Drosophila by an analogous mechanism during as during virus budding in human cells.
|
| Figure 1. Model of ALIX recruitment to the midbody in Drosophila melanogaster and human cells. ALIX (green) consists of an N-terminal Bro1-domain, a central V-domain and a C-terminal proline-rich region19. The hydrophobic ALIX-V-domain pocket is highlighted in dark green. During abscission in human cells (H. sapiens, bottom), the centralspindlin complex recruits the major midbody organizer CEP55 that directly binds and recruits ALIX and ESCRT-I6-8, 11-13, which in turn cooperatively recruit ESCRT-III7, 8, 14. ALIX interacts with the ESCRT-III subunit CHMP4B via its Bro1-domain and with CEP55 via its proline-rich region6-8, 10. However, CEP55 is missing in Drosophila melanogaster and other invertebrates, and it has remained unknown how ALIX is recruited to the midbody in the absence of CEP55. Lie-Jensen et al. elucidate that the centralspindlin subunit Pavarotti directly interacts via an LXXLF motif with the highly conserved hydrophobic pocket in the ALIX-V-domain to mediate Drosophila ALIX midbody recruitment and cytokinetic abscission15 (D. melanogaster, top). Drosophila ALIX also directly interacts with the ESCRT-III subunit Shrub (CHMP4B orthologue) to mediate cytokinetic abscission9. The image is from Lie-Jensen et al., Centralspindlin Recruits ALIX to the Midbody during Cytokinetic Abscission in Drosophila via a Mechanism Analogous to Virus Budding, Current Biology (2019), https://doi.org/10.1016/j.cub.2019.09.025. |
To date, only few physiological ligands for the ALIX-V-domain have been identified, and the work elucidates that Pavarotti is an endogenous ligand of the Drosophila ALIX-V-domain. Based on this study and findings in human cells, the centralspindlin complex emerges to play an ancestral role in recruiting the abscission machinery to the midbody. Future work will elucidate whether this mechanism of ALIX recruitment applies during cytokinesis in different species and cell types.
The work has been performed by Anette Lie-Jensen and members of the project group of Kaisa Haglund in the group of Harald Stenmark and researchers at the Department of Molecular Cell Biology at the Institute of Cancer Research and Centre for Cancer Cell Programming, in collaboration with Dr. Jon Lærdahl and Prof. Knut Liestøl at the Department of Informatics at the University of Oslo, who have performed bioinformatic and statistical analyses, respectively.
Links:
The article:
Centralspindlin Recruits ALIX to the Midbody during Cytokinetic Abscission in Drosophila via a Mechanism Analogous to Virus Budding.
Lie-Jensen A, Ivanauskiene K, Malerød L, Jain A, Tan KW, Laerdahl JK, Liestøl K, Stenmark H, Haglund K.
Curr Biol. 2019 Oct 7. pii: S0960-9822(19)31187-X. doi: 10.1016/j.cub.2019.09.025. [Epub ahead of print]
PMID: 31607533
Kaisa Haglund’s project group: Cytokinesis in development and carcinogenesis
Department of Molecular Cell Biology
Centre for Cancer Cell Programming
Previous news articles about Kaisa Haglund from ous-research.no:
Funding from FRIPRO to five promising researchers from Oslo University Hospital (25.01.2019)
Ragnar Mørk legacy prize 2018 to Kaisa Haglund (26.11.2018)
EMBO Journal article from Malerød and Haglund: Orienting the spindle (12.06.2018)
Jahre prize to Kaisa Haglund (12.10.2015)
Recognition of outstanding scientific work: - Kaisa Haglund receives the Jahre prize for young medical researchers (03.09.2015)
Kaisa Haglund awarded prestigious grant (19.01.2012)
Kaisa Haglund identifies novel regulator of cytokinesis (20.05.2010)
References
- Christ, L., Raiborg, C., Wenzel, E.M., Campsteijn, C. & Stenmark, H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci 42, 42-56 (2017).
- Raiborg, C. & Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445-452 (2009).
- Guizetti, J. et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 331, 1616-1620 (2011).
- Elia, N., Ott, C. & Lippincott-Schwartz, J. Incisive imaging and computation for cellular mysteries: lessons from abscission. Cell 155, 1220-1231 (2013).
- Caballe, A. & Martin-Serrano, J. ESCRT machinery and cytokinesis: the road to daughter cell separation. Traffic 12, 1318-1326 (2011).
- Carlton, J.G. & Martin-Serrano, J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316, 1908-1912 (2007).
- Morita, E. et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26, 4215-4227 (2007).
- Carlton, J.G., Agromayor, M. & Martin-Serrano, J. Differential requirements for Alix and ESCRT-III in cytokinesis and HIV-1 release. Proc Natl Acad Sci U S A 105, 10541-10546 (2008).
- Eikenes, A.H. et al. ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell Division In Vivo. Plos Genet 11 (2015).
- McCullough, J., Fisher, R.D., Whitby, F.G., Sundquist, W.I. & Hill, C.P. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc Natl Acad Sci U S A105, 7687-7691 (2008).
- Zhao, W.M., Seki, A. & Fang, G. Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell 17, 3881-3896 (2006).
- Lee, H.H., Elia, N., Ghirlando, R., Lippincott-Schwartz, J. & Hurley, J.H. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science 322, 576-580 (2008).
- Bastos, R.N. & Barr, F.A. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J Cell Biol 191, 751-760 (2010).
- Christ, L. et al. ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J Cell Biol 212, 499-513 (2016).
- Lie-Jensen, A. et al. Centralspindlin Recruits ALIX to the Midbody during Cytokinetic Abscission in Drosophila via a Mechanism Analogous to Virus Budding. Curr Biol (2019).
- Martin-Serrano, J. & Neil, S.J. Host factors involved in retroviral budding and release. Nat Rev Microbiol 9, 519-531 (2011).
- Ren, X. & Hurley, J.H. Proline-rich regions and motifs in trafficking: from ESCRT interaction to viral exploitation. Traffic 12, 1282-1290 (2011).
- Votteler, J. & Sundquist, W.I. Virus budding and the ESCRT pathway. Cell Host Microbe 14, 232-241 (2013).
- Odorizzi, G. The multiple personalities of Alix. J Cell Sci 119, 3025-3032 (2006).