ADOPTIVE T-CELL THERAPY (TCR)
Ideally, efficient tumour-specific effector and memory T cells can be induced by therapeutic vaccination. Nevertheless, in certain cases, active immunization is difficult due to the lack of an effective endogenous T-cell repertoire against the tumour antigens targeted.
T cells can recognize peptides derived from all parts of the cell, including nuclear proteins, which greatly expands the number of potential targets in the tumour cells and offers a large number of new therapeutic opportunities.
Adoptive cell therapy (ACT) involves the administration of large number of highly selected cells with high avidity for tumour antigens.
T cells can be programmed and activated ex vivo to exhibit anti-tumour functions. These cells occur naturally in cancer patients, but are inhibited by numerous immunosuppressive mechanisms in vivo. Adoptive transfer of tumour-specific T cells from tumour infiltrating lymphocytes (TIL) expanded in vitro has already been shown to induce objective cancer regression in patients with metastatic melanoma. A limitation of this approach is the requirement for pre-existing tumour-reactive cells that can be expanded ex vivo and TILs can only be reliably grown from patients with a very limited number of cancer types and mainly melanoma.
In the absence of an adequate level of endogenous tumour-reactive T-cell response, recent clinical studies have shown that it is feasible to compensate for this by engineering a tumour-specific T-cell repertoire by the transfer of genes encoding TCRs or chimeric antigen receptors (CARs). Both of these methods are developed in-house (see below).
T-cell receptors (PI: E.M. Inderberg):
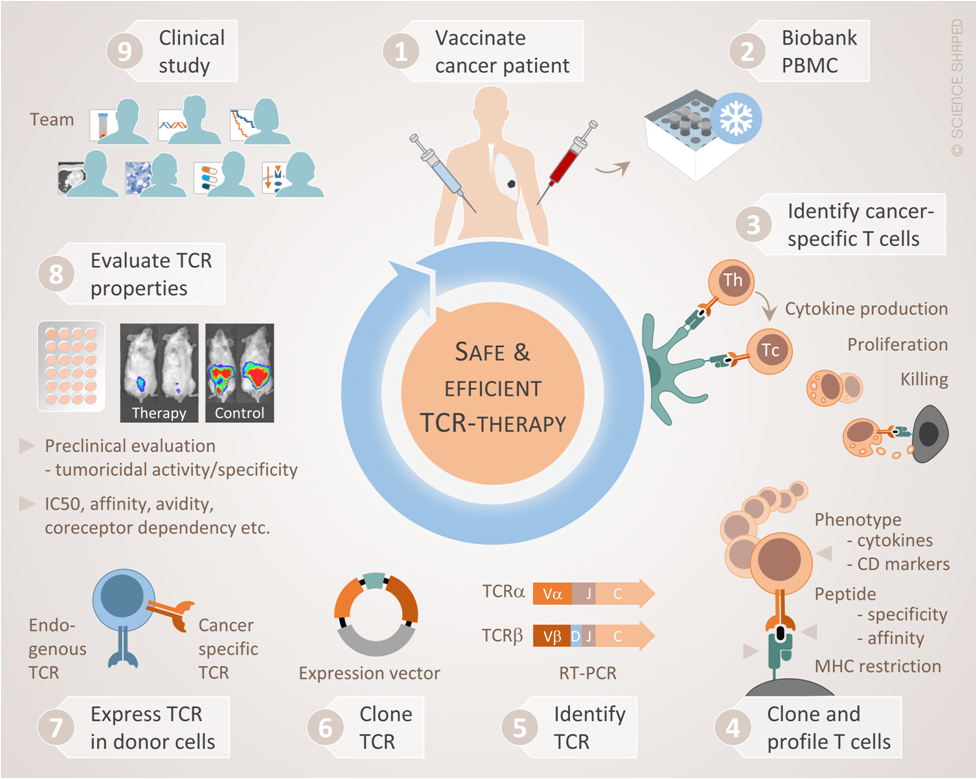
Early clinical trials have demonstrated that reprogrammed T cells may effectively eradicate cancer, even in patients with disseminated disease. These results have primarily been achieved in patients with certain hematological malignancies. In the present project, we aim at extending this therapeutic principle to most cancer forms, by targeting the universal tumour antigens such as telomerase or public neoantigens like TGFbR2 or KRAS mutants. Our research consortium at OUS-Radiumhospitalet has conducted a series of clinical trials with cancer vaccines demonstrating prolonged survival for patients that developed an immune response. However, only a minority of patients with advanced disease appear able to respond clinically after vaccination partly due to the cancer being too advanced and their immune system being suppressed by the tumour.
In the present project, we bypass the tumour’s success in escaping the host immune system, by equipping the patient’s T cells with tumour-targeting TCRs. We have isolated a selection of tumour-specific TCRs from long term survivors after cancer vaccination. These TCRs have circulated for up to 10 years in the patients, without causing side effects, and have been associated with clinical responses. In pre-clinical studies, the TCRs appear to effectively reprogram otherwise non-responsive T cells into tumour-targeting missiles. Starting from patients who have benefitted clinically from immunotherapy we have thus isolated tumour-reactive TCRs that could potentially be exploited to fight cancer in a large proportion of patients. Our strategy can be described as Clinic-to-bench-and-back-to-Clinic as illustrated below:
Funding from the Norwegian Research Council (BIOTEK2021 and FORNY) and Helse Sør-Øst Innovation grant.
This also formed the basis for the establishment of a start-up company, Zelluna Immunotherapy AS.