Welcome to the Radiation Biology and DNA Damage Signaling group.

|
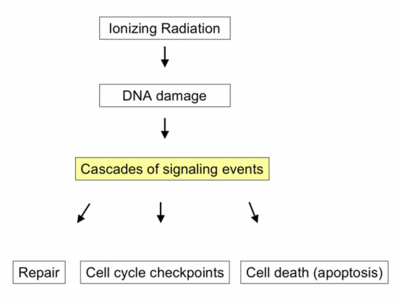 |
In radiotherapy, ionizing radiation eradicates cancer cells by causing DNA damage. However, in reaction to this damage, a network of intracellular DNA damage signaling pathways is activated. These pathways play a crucial role in determining tumor cell radiosensitivity, by modulating DNA repair mechanisms, cell cycle checkpoints, and cell death pathways. Intriguingly, recent studies suggest that these signaling pathways also regulate the radiation-induced anti-tumor immune response.
Our goal is to uncover novel mechanisms within these signaling pathways and understand how inhibitors targeting DNA damage signaling and repair can be utilized to improve cancer treatment.
Ongoing projects:
- Effects of DNA damage signaling inhibitors on anti-tumor immune responses after irradiation
- Drug repurposing screening with irradiation and drug libraries (endpoints: cell viability, DNA repair, G2 checkpoint)
- Pre-clinical studies of radiosensitizers combined with proton versus X-ray irradiation
- Regulation of replication stress and DNA damage signaling pathways by RNA polymerase II phosphatases
Methods:
We study human cancer and normal cells and use many different techniques including immunofluorescence microscopy and live cell imaging, multiparameter flow cytometry, immunoblotting, immunoprecipitation, protein overexpression, siRNA transfection, hypoxia treatment, X-ray irradiation, proton irradiation, and robot-automated cell viability and flow cytometry large-scale screening.
Selected group publications (2011-2024):
1. A. Eek Mariampillai, S. Hauge, K. Kongsrud, R.G. Syljuåsen. "Immunogenic cell death after combined treatment with radiation and ATR inhibitors is dually regulated by apoptotic caspases" Front Immunol, 14, 1138920, 2023.
2. L.T.E. Bay, R.G. Syljuåsen, H.B. Landsverk. "A novel, rapid and sensitive flow cytometry method reveals degradation of promoter proximal paused RNAPII in the presence and absence of UV" Nucleic Acids Res, 50 (15), e89, 2022.
3. G.E. Rødland, S. Hauge, G. Hasvold , L.T.E. Bay, T.H. Raabe, M. Joel, R.G. Syljuåsen. "Differential effects of combined ATR/WEE1 inhibition in cancer cells" Cancers (Basel), 13 (15), 2021.
4. HB. Landsverk, L.E. Sandquist, L.T.E. Bay, B. Steurer,C. Campsteijn, O.J.B. Landsverk, J.A. Marteijn, E. Petermann, L. Trinkle-Mulcahy, R.G. Syljuåsen. "WDR82/PNUTS-PP1 Prevents Transcription-Replication Conflicts by Promoting RNA Polymerase II Degradation on Chromatin" Cell Reports, 33(9):108469, 2020.
5. H.B. Landsverk, L.E. Sandquist, S.C. Sridhara, G.E. Rødland, J.C. Sabino, S.F. de Almeida, B. Grallert, L. Trinkle-Mulcahy, R.G. Syljuåsen. "Regulation of ATR activity via the RNA polymerase II associated factors CDC73 and PNUTS-PP1" Nucleic Acids Research, 47, p.1797-1813, 2019.
6. S. Hauge, C. Naucke, G. Hasvold, M. Joel, G.E. Rødland, P. Juzenas, T. Stokke, R.G. Syljuåsen. "Combined inhibition of Wee1 and Chk1 gives synergistic DNA damage in S-phase due to distinct regulation of CDK activity and CDC45 loading" Oncotarget, 8, p.10966-10979, 2017.
7. T.W. Håland, E. Boye, T. Stokke, B. Grallert, R.G. Syljuåsen. "Simultaneous measurement of passage through the restriction point and MCM loading in single cells" Nucleic Acids Research, 43, p.e150, 2015.
8. C. Lund-Andersen, S. Patzke, V. Nähse-Kumpf, R.G. Syljuåsen. "Plk1-inhibition can cause radiosensitization or radioresistance dependent on the treatment schedule" Radiation Therapy and Oncology, 110, p.355-361, 2014.
9. H.Beck1,V. Nähse-Kumpf1, M.S Yoo Larsen, S. Patzke, C. Holmberg, O. Nielsen, R.G. Syljuåsen*, C.S. Sørensen*. "CDK suppression by WEE1 kinase protects the genome through control of replication initiation and nucleotide consumption" Mol. Cell. Biol., 32, 4226-36, 2012. 1Equal contribution.
10. T. Menzel, V. Nähse-Kumpf, A. Nedergaard Kousholt, D. Kjærsgaard Klein, C. Lund-Andersen, M. Lees, J. Vilstrup Johansen, R.G. Syljuåsen*, C.S. Sørensen*. "A genetic screen identifies BRCA2 and PALB2 as key regulators of G2 checkpoint maintenance" EMBO Reports, 12, p.705-712, 2011.
Contact information:
Randi G. Syljuåsen, Department of Radiation Biology, Institute for Cancer Research, Norwegian Radium Hospital, Oslo University Hospital. Phone: +47 22781468. email: randi.syljuasen@rr-research.no